
Describe an experiment to demonstrate that air exerts pressure.
Answer
589.8k+ views
Hint: The weight of the air acting on a unit area of the surface is known as air pressure or atmospheric pressure. Air exerts pressure in all directions. This atmospheric pressure causes air molecules at the surface of the earth to be more tightly packed together than those that are high in the atmosphere.
Complete step by step solution:
Experiment:
The Collapsing Can
Materials needed:
1. One empty can, or any other tin can that can be closed off air-tight.
2. A burner or hot plate and tripod.
Strategy:
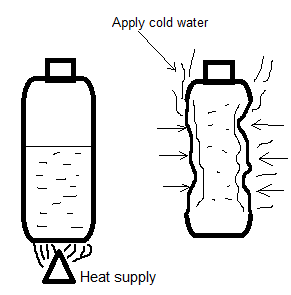
1. Fill $20ml$ of water in the can (To cover the bottom) and heat it over the burner or hot plate.
2. Boil the water vigorously for about $2\min $ (vapors should come out of the can).
3. Take out the can from the heat with the boiling water and immediately close the can with the cap very tightly.
4. Keep the can upright on the table and cool off the can room temperature, or, to cool it faster, cool off with a wet towel.
Conclusion:
When the air is pushed out by the water vapor (which occupied the interior space of the can) after heating was allowed to cool, its volume is reduced by approximately $1000$. When the volume gets decreased inside the can, then the outside pressure crushed the can. The force working on the outside of the can is the total of the can's surface area in $c{m^2}$ multiplied by $1\,kg$.
Note:
A barometer is commonly used to measure the air pressure. The first barometer was developed in $1600\,s$. The barometer working is done by balancing the weight of mercury in the glass tube against the atmospheric pressure, much like a set of scales. Two main types used in meteorology are the mercury barometer and the aneroid barometer.
Complete step by step solution:
Experiment:
The Collapsing Can
Materials needed:
1. One empty can, or any other tin can that can be closed off air-tight.
2. A burner or hot plate and tripod.
Strategy:
1. Fill $20ml$ of water in the can (To cover the bottom) and heat it over the burner or hot plate.
2. Boil the water vigorously for about $2\min $ (vapors should come out of the can).
3. Take out the can from the heat with the boiling water and immediately close the can with the cap very tightly.
4. Keep the can upright on the table and cool off the can room temperature, or, to cool it faster, cool off with a wet towel.

Conclusion:
When the air is pushed out by the water vapor (which occupied the interior space of the can) after heating was allowed to cool, its volume is reduced by approximately $1000$. When the volume gets decreased inside the can, then the outside pressure crushed the can. The force working on the outside of the can is the total of the can's surface area in $c{m^2}$ multiplied by $1\,kg$.
Note:
A barometer is commonly used to measure the air pressure. The first barometer was developed in $1600\,s$. The barometer working is done by balancing the weight of mercury in the glass tube against the atmospheric pressure, much like a set of scales. Two main types used in meteorology are the mercury barometer and the aneroid barometer.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
What is BLO What is the full form of BLO class 8 social science CBSE

Citizens of India can vote at the age of A 18 years class 8 social science CBSE

Full form of STD, ISD and PCO

Advantages and disadvantages of science

Right to vote is a AFundamental Right BFundamental class 8 social science CBSE

What are the 12 elements of nature class 8 chemistry CBSE




