
Describe simple experiment to prove that gases occupy space
Answer
508.8k+ views
Hint:One of the four basic states of matter is gas (the others being solid, liquid, and plasma). A pure gas can be made up of individual atoms (such as a noble gas like neon), elemental molecules (such as oxygen), or complex molecules (composed of a variety of atoms) (e.g. carbon dioxide).
Complete answer:
A gas mixture, such as air, consists of a number of different pure gases. The great separation of individual gas particles separates a gas from liquids and solids. A colourless gas is normally undetectable to a human observer due to this gap. Between the liquid and plasma phases, the gaseous state of matter exists, with the latter serving as the upper temperature barrier for gases. Degenerate quantum gases, which exist at the lower end of the temperature scale, are rising in popularity. High-density atomic gases that have been supercooled to extremely low temperatures are classed as either Bose gases or Fermi gases based on their statistical behaviour.
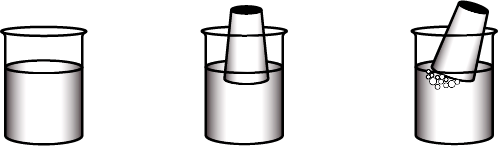
Water is half-filled in a glass bowl. Inside the bowl, an empty glass tumbler is inverted. Bubbles appear in the water when the tumbler is tilted. This indicates that air was filling space inside the tumbler, and that when the tumbler is tilted, the air is displaced, causing bubbles to emerge.
Note:
It is common practise to designate a frame of reference or length scale while viewing a gas. A greater length scale correlates to the gas's macroscopic or global perspective. This area (also known as a volume) must be large enough to hold a large sample of gas particles. The "average" behaviour (i.e. velocity, temperature, or pressure) of all the gas particles inside the area is determined by the statistical analysis of this sample size. A lower length scale, on the other hand, corresponds to a microscopic or particulate point of view.
Complete answer:
A gas mixture, such as air, consists of a number of different pure gases. The great separation of individual gas particles separates a gas from liquids and solids. A colourless gas is normally undetectable to a human observer due to this gap. Between the liquid and plasma phases, the gaseous state of matter exists, with the latter serving as the upper temperature barrier for gases. Degenerate quantum gases, which exist at the lower end of the temperature scale, are rising in popularity. High-density atomic gases that have been supercooled to extremely low temperatures are classed as either Bose gases or Fermi gases based on their statistical behaviour.
Water is half-filled in a glass bowl. Inside the bowl, an empty glass tumbler is inverted. Bubbles appear in the water when the tumbler is tilted. This indicates that air was filling space inside the tumbler, and that when the tumbler is tilted, the air is displaced, causing bubbles to emerge.

Note:
It is common practise to designate a frame of reference or length scale while viewing a gas. A greater length scale correlates to the gas's macroscopic or global perspective. This area (also known as a volume) must be large enough to hold a large sample of gas particles. The "average" behaviour (i.e. velocity, temperature, or pressure) of all the gas particles inside the area is determined by the statistical analysis of this sample size. A lower length scale, on the other hand, corresponds to a microscopic or particulate point of view.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




