
How would you describe the arrangement of sodium ions $N{a^ + }$and chloride ions $C{l^ - }$in a crystal of sodium chloride $NaCl$?
Answer
558k+ views
Hint: Take help of types of unit cell, which are of three types simple cubic, body centred and face centred. These unit cells are a small portion of three dimensional lattice, when we rotate the unit cells all around it will give us a lattice. Here ions are arranged in such a way that crystals of sodium chloride $NaCl$ have FCC type of arrangement.
Complete step-by-step answer:
In the chapter on solid state, it gives us an idea of how the complex structures are arranged. A lattice is formed by the number of atoms arranged in a small unit cell. Unit cells have sides which may be equal or not. Famously we talk a lot about the cubic cell type. These cells are such that the position of atoms or ions matters. We have three types of cubic unit cells. The first one is the simple cubic in this type. The particles are just at the corner and you can imagine that while sitting in your room. So as there are eight corners in a cube so there are eight atoms in a simple cubic unit cell.
In the other two that are body centred and face centred, the atoms are arranged including eight corners and also at the body centred which is just between the cube and in face centred atoms are also present at each of the face including the corners.
The lattice crystal given of sodium chloride $NaCl$ , a salt which we used for cooking, has FCC face centred crystal lattice and contains a unit cell in which there are atoms at the corners and also at each face. In the unit cell there are voids which are present, in which the particles take its site. So in sodium chloride $NaCl$ crystal.
We have a lattice which is formed by chloride ions $C{l^ - }$ present at the corners and sodium ions which are present in the voids. Imagine such that the chloride ions $C{l^ - }$ which are bigger in size are present at the corners of a cube and all other sodium ions $N{a^ + }$ are at each face centred site.
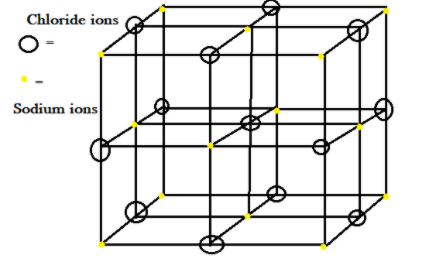
Note: To understand any crystal lattice, consider a small part i.e. unit cell where the atoms are arranged. In the above example of $NaCl$, why chloride ions $C{l^ - }$ formed lattice and all sodium ions $N{a^ + }$ are present at a smaller void site? This arrangement is possible and decided by the radius ratio rule, thus we can say cation decides that in which void it wants to live formed by arrangement of anions.
Complete step-by-step answer:
In the chapter on solid state, it gives us an idea of how the complex structures are arranged. A lattice is formed by the number of atoms arranged in a small unit cell. Unit cells have sides which may be equal or not. Famously we talk a lot about the cubic cell type. These cells are such that the position of atoms or ions matters. We have three types of cubic unit cells. The first one is the simple cubic in this type. The particles are just at the corner and you can imagine that while sitting in your room. So as there are eight corners in a cube so there are eight atoms in a simple cubic unit cell.
In the other two that are body centred and face centred, the atoms are arranged including eight corners and also at the body centred which is just between the cube and in face centred atoms are also present at each of the face including the corners.
The lattice crystal given of sodium chloride $NaCl$ , a salt which we used for cooking, has FCC face centred crystal lattice and contains a unit cell in which there are atoms at the corners and also at each face. In the unit cell there are voids which are present, in which the particles take its site. So in sodium chloride $NaCl$ crystal.
We have a lattice which is formed by chloride ions $C{l^ - }$ present at the corners and sodium ions which are present in the voids. Imagine such that the chloride ions $C{l^ - }$ which are bigger in size are present at the corners of a cube and all other sodium ions $N{a^ + }$ are at each face centred site.

Note: To understand any crystal lattice, consider a small part i.e. unit cell where the atoms are arranged. In the above example of $NaCl$, why chloride ions $C{l^ - }$ formed lattice and all sodium ions $N{a^ + }$ are present at a smaller void site? This arrangement is possible and decided by the radius ratio rule, thus we can say cation decides that in which void it wants to live formed by arrangement of anions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




