
α-D-Glucose and β-D-Glucose are not –
(A) Epimers
(B) Anomers
(C) Enantiomers
(D) Diastereomers
Answer
564.9k+ views
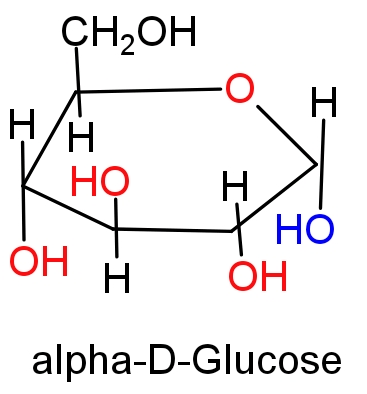
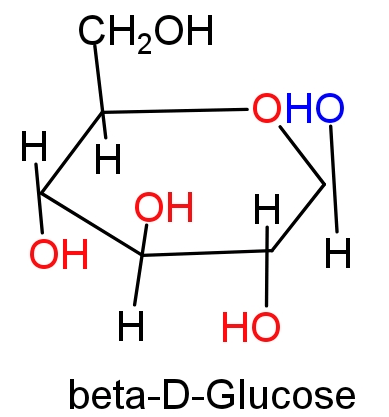
Hint: Glucose forms a six membered cyclic ring known as a pyranose ring in which $\text{-OH}$group at $\text{C-5}$ is involved in ring formation. In intramolecular hemiacetal formation $\text{-CHO}$ group of glucose is converted into $\text{CHOH}$ which can have two configurations on the bases of position of $\text{-OH}$ group at$\text{C-1}$; α-D-Glucose and β-D-Glucose. Both the forms of glucose is positional isomers of each other at $\text{C-1}$ position and this carbon is known as anomeric carbon.
Complete answer:
(A) Those diastereomers which have identical configuration at all the chiral centres other than anomeric carbon are called epimers. For e.g. glucose and mannose are differ from each other in the configuration of $\text{C-2}$ hence they are called ${{\text{C}}_{\text{4}}}$ epimers. Since, α-D-Glucose and β-D-Glucose only differ in $\text{C-1}$ carbon configuration, these monosaccharides cannot be epimers. Hence option (A) will be the correct answer.
(B) Those cyclic diastereomers which differ in the configuration of $\text{C-1}$ carbon of glucose and reset of the molecule have identical configuration are called anomers. Hence α and β forms of glucose are anomers.
(C) Enantiomers are non- superimposable mirror images of an organic compound, since α and β form of glucose are non-superimposable mirror images of each other, hence these monosaccharides are enantiomers.
(D) Diastereomers are non- superimposable and are not mirror images of an organic compound, since α and β forms of glucose are non-superimposable and non- mirror images of each other, hence these monosaccharides are diastereomers .
Note:
Isomers are those chemical compounds which have the same molecular formula but they differ either in the position or types of functional group. Enantiomers and diastereomers are the only defined form optically active compounds.
D and L configuration of monosaccharides is assigned on the bases of the lowest asymmetrical carbon atom of sugar molecule.
In α-D-Glucose represents the cis- form of $\text{-OH}$ group and β-D-Glucose represents the trans- form of $\text{-OH}$ group in ${{\text{C}}_{\text{1}}}\text{-}{{\text{C}}_{\text{2}}}$ bond.


Complete answer:
(A) Those diastereomers which have identical configuration at all the chiral centres other than anomeric carbon are called epimers. For e.g. glucose and mannose are differ from each other in the configuration of $\text{C-2}$ hence they are called ${{\text{C}}_{\text{4}}}$ epimers. Since, α-D-Glucose and β-D-Glucose only differ in $\text{C-1}$ carbon configuration, these monosaccharides cannot be epimers. Hence option (A) will be the correct answer.
(B) Those cyclic diastereomers which differ in the configuration of $\text{C-1}$ carbon of glucose and reset of the molecule have identical configuration are called anomers. Hence α and β forms of glucose are anomers.
(C) Enantiomers are non- superimposable mirror images of an organic compound, since α and β form of glucose are non-superimposable mirror images of each other, hence these monosaccharides are enantiomers.
(D) Diastereomers are non- superimposable and are not mirror images of an organic compound, since α and β forms of glucose are non-superimposable and non- mirror images of each other, hence these monosaccharides are diastereomers .
Note:
Isomers are those chemical compounds which have the same molecular formula but they differ either in the position or types of functional group. Enantiomers and diastereomers are the only defined form optically active compounds.
D and L configuration of monosaccharides is assigned on the bases of the lowest asymmetrical carbon atom of sugar molecule.
In α-D-Glucose represents the cis- form of $\text{-OH}$ group and β-D-Glucose represents the trans- form of $\text{-OH}$ group in ${{\text{C}}_{\text{1}}}\text{-}{{\text{C}}_{\text{2}}}$ bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




