
Why did Mendeleev leave some gaps in his periodic table of elements?
Answer
584.4k+ views
Hint: In Mendeleev’s periodic table the elements are arranged in the order of their increasing relative atomic mass. When he arranged the atoms he noticed that the chemical properties of the present elements showed a periodic trend.
Complete step by step answer:
In 1869, Mendeleev’s a Russian chemist proposed a tabular diagram of known elements. At that time only 63 elements were known. He arranges the elements on the basis of their increasing atomic mass. But the most interesting part of Mendeleev's periodic table is that he left some spaces for the elements that were not discovered yet, and also predicted what will be the atomic mass and chemical properties of these elements.
Mendeleev arranged the elements in groups and periods. If we move from left to right in a row the elements are arranged by increasing atomic mass. He discovered that if he placed the 8 elements in each row and then continued placing the elements from the next row then the columns would contain elements having similar properties. He named the columns groups. The term periods are the rows of the Mendeleev’s periodic table.
For example- Mendeleev predicted the missing element in the row 5 of group 3. Mendeleev also predicted that the atomic number of the missing element will be 68 and it will be relatively soft metal. Scientists found that element and named it gallium. And in this way scientists found all the missing elements predicted by Mendeleev.
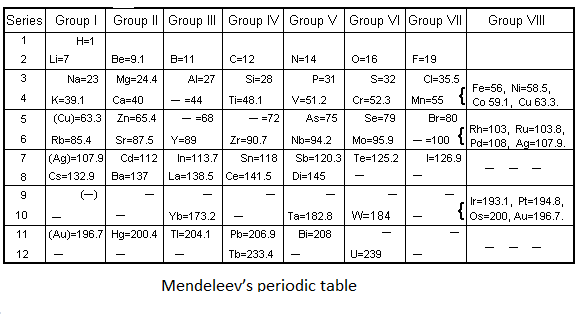
Note: Mendeleev’s predicted the existence of elements and named them eka-aluminium, eka-boron and eka-silicon, which was later named as gallium, scandium and germanium. He predicted the correct atomic mass of each of the elements and also the compounds each should form.
Complete step by step answer:
In 1869, Mendeleev’s a Russian chemist proposed a tabular diagram of known elements. At that time only 63 elements were known. He arranges the elements on the basis of their increasing atomic mass. But the most interesting part of Mendeleev's periodic table is that he left some spaces for the elements that were not discovered yet, and also predicted what will be the atomic mass and chemical properties of these elements.
Mendeleev arranged the elements in groups and periods. If we move from left to right in a row the elements are arranged by increasing atomic mass. He discovered that if he placed the 8 elements in each row and then continued placing the elements from the next row then the columns would contain elements having similar properties. He named the columns groups. The term periods are the rows of the Mendeleev’s periodic table.
For example- Mendeleev predicted the missing element in the row 5 of group 3. Mendeleev also predicted that the atomic number of the missing element will be 68 and it will be relatively soft metal. Scientists found that element and named it gallium. And in this way scientists found all the missing elements predicted by Mendeleev.

Note: Mendeleev’s predicted the existence of elements and named them eka-aluminium, eka-boron and eka-silicon, which was later named as gallium, scandium and germanium. He predicted the correct atomic mass of each of the elements and also the compounds each should form.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




