
What is the difference between acetaldehyde and benzaldehyde?
Answer
517.5k+ views
Hint: We have to remember that acetaldehyde and benzaldehyde have the same functional group that is aldehyde. The reactivity of both the compounds differs in nature. The chemical reactivity for both aldehydes is different. Both are organic compounds since they contain carbon atoms in them.
Complete answer:
We will look at some points to differentiate between acetaldehyde and benzaldehyde.
Benzaldehyde is an organic compound that is made up of carbon atoms arranged in a cyclic structure forming a benzene like molecule and an aldehyde group is attached to it whereas in acetaldehyde it is made up of methyl groups attached to it an aldehyde group.
An aromatic aldehyde is benzaldehyde and acetaldehyde is an aliphatic aldehyde.
Both are colorless liquid but the difference comes in the color of the liquid, benzaldehyde is light brownish in color and the other is colorless in appearance.
Acetaldehyde is less dense than water whereas the other is denser than water.
Benzaldehyde has a higher boiling point than acetaldehyde.
Solubility in water can also be a point of difference between these two. Acetaldehyde is miscible with water whereas benzaldehyde is not soluble in water.
We can draw the structure of benzaldehyde as,
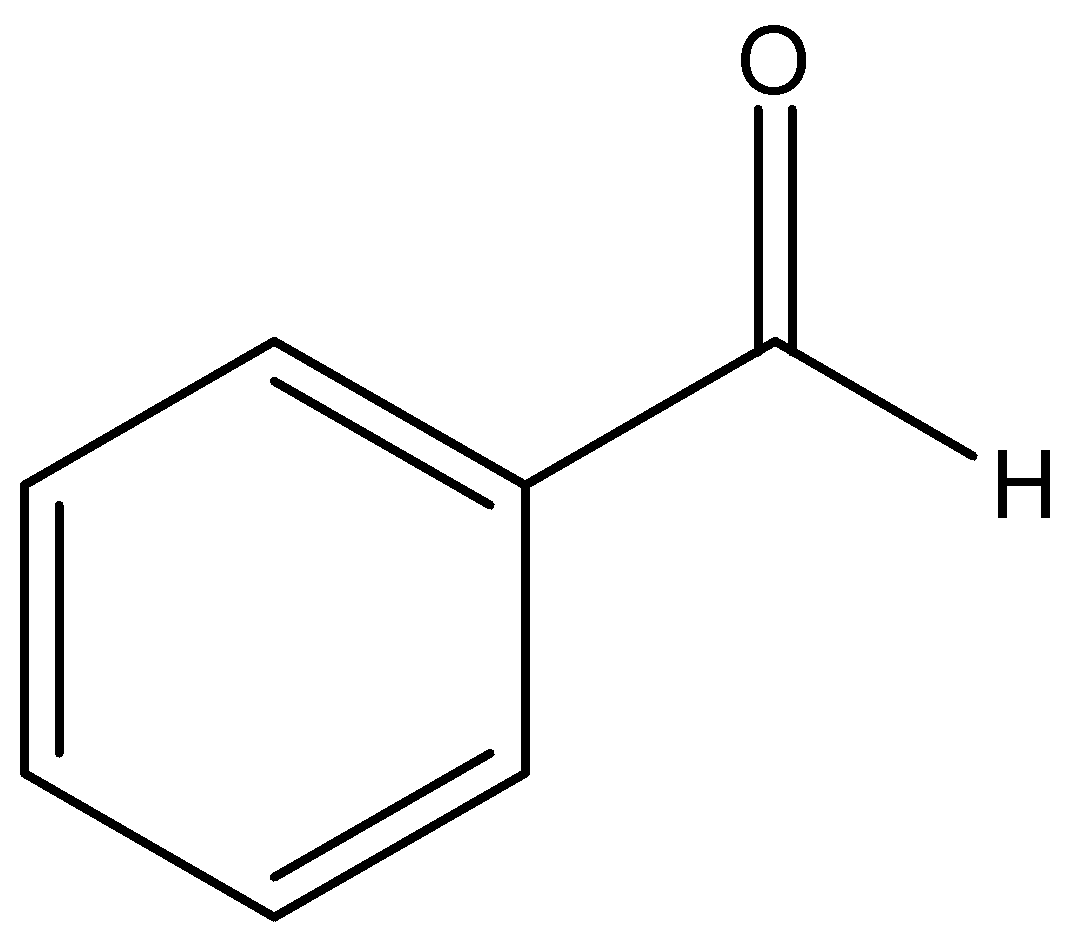
We can draw the structure of acetaldehyde:
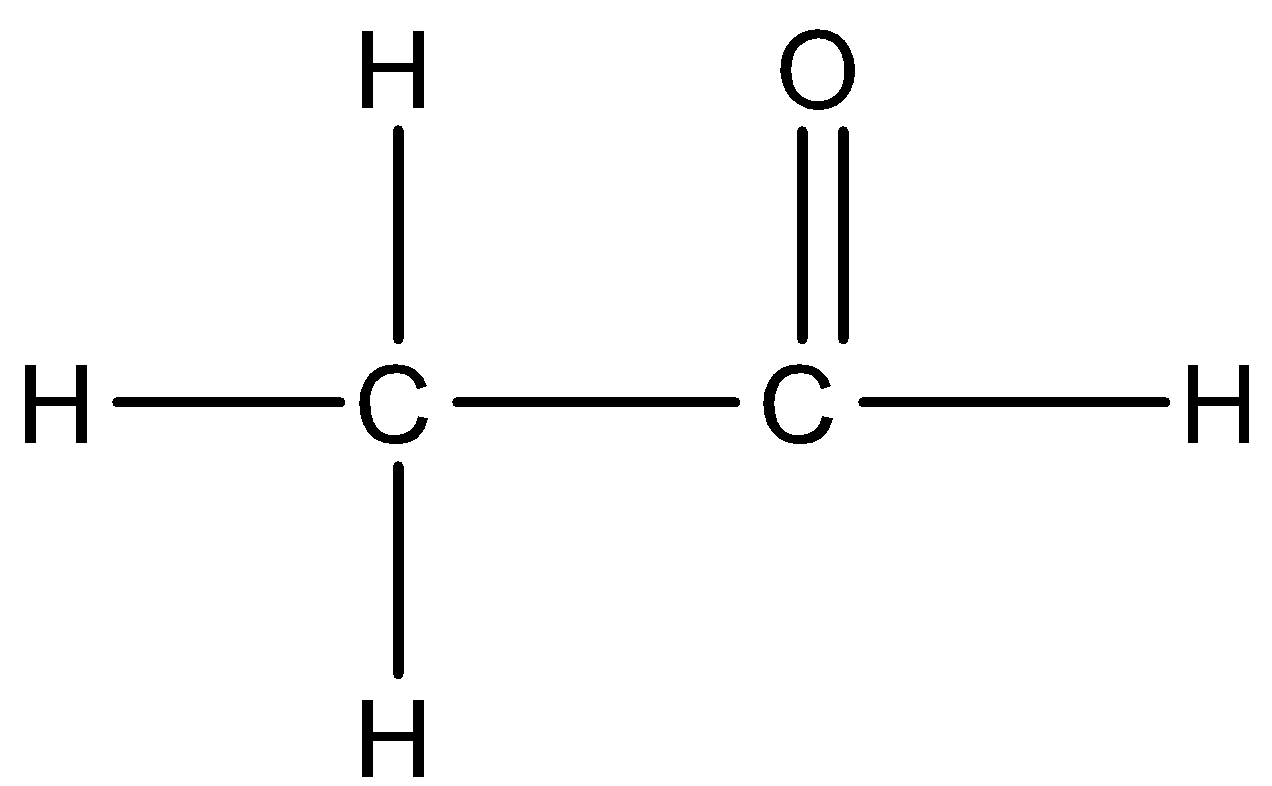
Note:
We have to know that both the compounds contain an aldehyde functional group that is \[ - CHO\]. Aromatic compounds can be identified easily as they contain a benzene ring in it while aliphatic compounds are only composed of methyl groups. Aldehydes can react with tollens reagent and give positive tests while benzaldehyde does not show this nature therefore we can say that reactivity for both is also different.
Complete answer:
We will look at some points to differentiate between acetaldehyde and benzaldehyde.
Benzaldehyde is an organic compound that is made up of carbon atoms arranged in a cyclic structure forming a benzene like molecule and an aldehyde group is attached to it whereas in acetaldehyde it is made up of methyl groups attached to it an aldehyde group.
An aromatic aldehyde is benzaldehyde and acetaldehyde is an aliphatic aldehyde.
Both are colorless liquid but the difference comes in the color of the liquid, benzaldehyde is light brownish in color and the other is colorless in appearance.
Acetaldehyde is less dense than water whereas the other is denser than water.
Benzaldehyde has a higher boiling point than acetaldehyde.
Solubility in water can also be a point of difference between these two. Acetaldehyde is miscible with water whereas benzaldehyde is not soluble in water.
We can draw the structure of benzaldehyde as,

We can draw the structure of acetaldehyde:

Note:
We have to know that both the compounds contain an aldehyde functional group that is \[ - CHO\]. Aromatic compounds can be identified easily as they contain a benzene ring in it while aliphatic compounds are only composed of methyl groups. Aldehydes can react with tollens reagent and give positive tests while benzaldehyde does not show this nature therefore we can say that reactivity for both is also different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




