
What is the difference between atomization energy and bond dissociation energy?
Answer
485.4k+ views
Hint: All that you must know is what is meant by atomization energy and bond dissociation energy.
In this, we can see what is meant by atomization energy and bond dissociation energy and their concepts.
Also, we can see the difference between atomization energy and bond dissociation energy so that you can understand them easily.
Complete answer:
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required they are the most important difference between the enthalpy of atomization and bond dissociation
Sometimes if there are simple bonds for some simple compounds it is the same for, enthalpy of atomization and enthalpy of bond dissociation. This is because the dissociation of bonds forms the atoms from which the simple compound is made of the molecule.
Enthalpy of Atomization Vs Bond Dissociation
For some simple compounds, bond dissociation and enthalpy of atomization can be interchangeable but they are not always equivalent.
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required
Atomization energy:
The enthalpy of atomization of either a chemical compound or a chemical element is The enthalpy change that accompanies the total separation of all atoms in a chemical substance.
It is denoted as $\vartriangle {H_{at}}$.
Bond dissociation energy:
Bond dissociation of enthalpy describes during the chemical bond dissociation where the enthalpy changes occur. In other words, the chemical bond is the measure of strength. Therefore, we can say when chemical bond$A - B$breaks down by hemolysis and fragments A and B is the enthalpy of bond dissociation when the stood enthalpy change occurs. The bond dissociation enthalpy is equal to the atomization enthalpy when the molecule that we are considering is a diatomic molecule. Usually, fragments of A and B given by this bond dissociation are radical species. We can denote $D{H^ \circ }$as the enthalpy of bond dissociation.
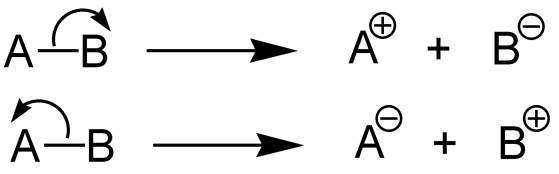
In bond dissociation, there are different methods we can use such as spectrometric determination of energy levels, generation of radicals by photolysis or pyrolysis, measurements of equilibrium and chemical kinetics, various calorimetric and electrochemical methods, etc…
Note:
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required they are the most important difference between the enthalpy of atomization and bond dissociation
Sometimes if there are simple bonds for some simple compounds it is the same for, enthalpy of atomization and enthalpy of bond dissociation. This is because the dissociation of bonds forms the atoms from which the simple compound is made of the molecule.
In this, we can see what is meant by atomization energy and bond dissociation energy and their concepts.
Also, we can see the difference between atomization energy and bond dissociation energy so that you can understand them easily.
Complete answer:
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required they are the most important difference between the enthalpy of atomization and bond dissociation
Sometimes if there are simple bonds for some simple compounds it is the same for, enthalpy of atomization and enthalpy of bond dissociation. This is because the dissociation of bonds forms the atoms from which the simple compound is made of the molecule.
Enthalpy of Atomization Vs Bond Dissociation
For some simple compounds, bond dissociation and enthalpy of atomization can be interchangeable but they are not always equivalent.
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required
Atomization energy:
The enthalpy of atomization of either a chemical compound or a chemical element is The enthalpy change that accompanies the total separation of all atoms in a chemical substance.
It is denoted as $\vartriangle {H_{at}}$.
Bond dissociation energy:
Bond dissociation of enthalpy describes during the chemical bond dissociation where the enthalpy changes occur. In other words, the chemical bond is the measure of strength. Therefore, we can say when chemical bond$A - B$breaks down by hemolysis and fragments A and B is the enthalpy of bond dissociation when the stood enthalpy change occurs. The bond dissociation enthalpy is equal to the atomization enthalpy when the molecule that we are considering is a diatomic molecule. Usually, fragments of A and B given by this bond dissociation are radical species. We can denote $D{H^ \circ }$as the enthalpy of bond dissociation.

In bond dissociation, there are different methods we can use such as spectrometric determination of energy levels, generation of radicals by photolysis or pyrolysis, measurements of equilibrium and chemical kinetics, various calorimetric and electrochemical methods, etc…
Note:
The bond dissociation of enthalpy describes chemical bonds of dissociation in a molecule whereas the enthalpy of atomization describes separating a molecule into its atoms the energy required they are the most important difference between the enthalpy of atomization and bond dissociation
Sometimes if there are simple bonds for some simple compounds it is the same for, enthalpy of atomization and enthalpy of bond dissociation. This is because the dissociation of bonds forms the atoms from which the simple compound is made of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




