
What is the difference between Keesom interactions and London dispersion forces?
Answer
478.5k+ views
Hint: Intermolecular forces are the forces that conciliate between molecules, including forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. The intermolecular forces are weaker than intermolecular forces.
Complete answer:
The Difference between Keesom Interaction and London dispersion forces are as follows:
Note:
Remember dispersion forces are present between all molecules, whether they are polar or nonpolar whereas Keesom interaction occurs only in polar molecules. The London dispersion forces tend to be stronger between molecules that are easily polarized whereas weaker between the molecules that are not easily polarized.
Complete answer:
The Difference between Keesom Interaction and London dispersion forces are as follows:
| Keesom Interaction | London dispersion Forces |
| The Keesom interaction happens from a permanent dipole in another permanent dipole. It is the electrostatic interaction among the permanent dipoles of polar molecules. This can be conjointly referred to as permanent dipole-dipole force. | The London dispersion force could be a non-permanent attraction that happens when the electrons in $2$ adjacent atoms occupy positions that make the atoms form non-permanent dipoles. This can be conjointly referred to as induced dipole-induced dipole attraction. |
| The Keesom interactions are stronger than London dispersion forces in small molecules. But in larger molecules, Keesom interactions are weaker than London dispersion forces. | In chemical bonding, the weakest intermolecular force is the London dispersion force. But in larger molecules, London dispersion forces are stronger than Keesom interactions. |
| Keesom interaction is that the result of $2$ permanent dipoles that arise between $2$ two polar molecules. | The London dispersion force is that the result of non-permanent dipole moments caused by asymmetrical distribution of electrons. |
Keesom interactions occur when induced negative end 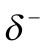
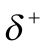
| In London dispersion force, an atom or molecule will develop a non-permanent dipole due to the constant motion of electrons, once its electrons are distributed asymmetrically concerning the nucleus. |
Note:
Remember dispersion forces are present between all molecules, whether they are polar or nonpolar whereas Keesom interaction occurs only in polar molecules. The London dispersion forces tend to be stronger between molecules that are easily polarized whereas weaker between the molecules that are not easily polarized.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




