
What is the difference between multimolecular and macromolecular colloids? Give one example each. How are associated colloids different from these types of colloids?
Answer
529.7k+ views
Hint: In colloidal state, the size of the particles varies from 1 nm to 100 nm (or\[{{10}^{-9\text{ }}}\text{to 1}{{\text{0}}^{-7}}\text{ m}\]). If some insoluble solid particles are dispersed in a medium such that after dispersion their particle size lies in the colloidal range then depending upon the way of forming colloidal size particles, these dispersion mediums are called as multimolecular, macromolecular and associated colloids.
Complete step by step solution:
Differences between multimolecular and macromolecular colloids:
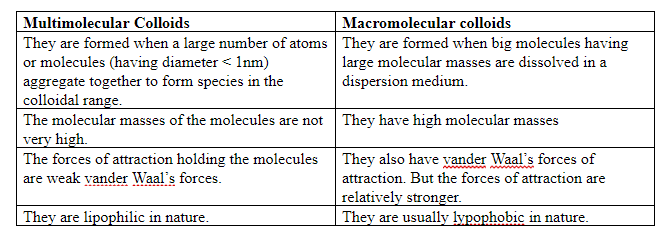
Examples of multimolecular colloid:
-Gold sol and sulphur sol consist of thousands of particles held together by vander Waal’s forces.
Examples of macromolecular colloids:
-Macromolecules like starch, cellulose, proteins, polymer like rubber, and gelatin. These molecules have sizes in the colloidal range and their dispersion forms macromolecular colloids.
-Associated colloids are different from multimolecular and macromolecular colloids in the way as follows:
Substances when dissolved in a medium at low concentrations behave as normal electrolytes but at higher concentration aggregate to form a micelle are associated colloids. The narrow concentration range over which the physicochemical properties of particles change due to the formation of oriented colloidal aggregates is called critical micelle concentration (CMC) and the aggregates formed are called micelles. For example: surface active agents like soaps and detergents.
No micelle formation takes place in either of the two colloids i.e. multimolecular and macromolecular colloids.
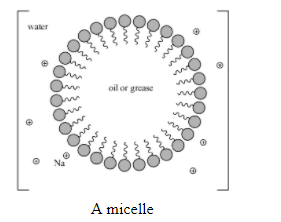
Additional Information: The formation of micelle takes place at a particular temperature called Kraft temperature (\[{{T}_{k}}\]).
Note: Carefully note the characteristics and conditions for all the three types of colloids. Students are more likely to get confused between multimolecular and macromolecular colloids. Multi means many so, in multimolecular many different particles combine to give a colloidal size particle whereas in macromolecular colloids, individual particles have colloidal size.
Complete step by step solution:
Differences between multimolecular and macromolecular colloids:

Examples of multimolecular colloid:
-Gold sol and sulphur sol consist of thousands of particles held together by vander Waal’s forces.
Examples of macromolecular colloids:
-Macromolecules like starch, cellulose, proteins, polymer like rubber, and gelatin. These molecules have sizes in the colloidal range and their dispersion forms macromolecular colloids.
-Associated colloids are different from multimolecular and macromolecular colloids in the way as follows:
Substances when dissolved in a medium at low concentrations behave as normal electrolytes but at higher concentration aggregate to form a micelle are associated colloids. The narrow concentration range over which the physicochemical properties of particles change due to the formation of oriented colloidal aggregates is called critical micelle concentration (CMC) and the aggregates formed are called micelles. For example: surface active agents like soaps and detergents.
No micelle formation takes place in either of the two colloids i.e. multimolecular and macromolecular colloids.

Additional Information: The formation of micelle takes place at a particular temperature called Kraft temperature (\[{{T}_{k}}\]).
Note: Carefully note the characteristics and conditions for all the three types of colloids. Students are more likely to get confused between multimolecular and macromolecular colloids. Multi means many so, in multimolecular many different particles combine to give a colloidal size particle whereas in macromolecular colloids, individual particles have colloidal size.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




