
What is the difference in electronegativity between magnesium and chlorine when they form to react to form magnesium chloride?
Answer
524.1k+ views
Hint: Before answering this question, we should first know what is electronegativity. The ability of an atom to attract the electrons that are being shared to itself is known as electronegativity. It depends on the atomic number as well as the distance at which its outermost electron lies from the nucleus.
Complete answer:
Magnesium belongs to Group-2 i.e Alkaline earth metals whereas chlorine is a non-metal that belongs to Group-7 i.e Halogens. The two elements do not share electrons, So there is no covalent bonding between them.
The magnesium atom gives away two electrons and forms a stable 2+ ion of magnesium whereas the chlorine atom accepts the electron and forms a stable 1- ion of chlorine which results in the formation of an ionic bond.
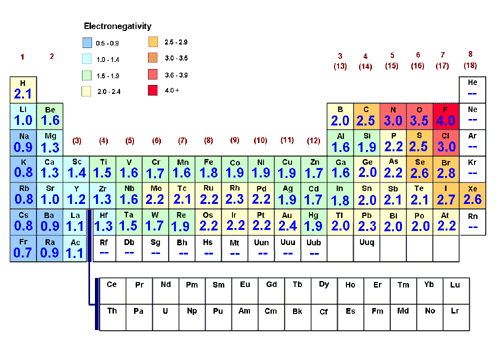
The electronegativity of two elements are as follows:
Magnesium- 1.31
Chlorine- 3.16
We have to find out the difference in their electronegativities, we can subtract the smaller value of electronegativity from the bigger value of electronegativity.
\[\Delta En=E{{n}_{Cl}}-E{{n}_{Mg}}\]
$\Delta En=3.16-1.31$
$\vartriangle En=1.85$
Here ionic bonding happens because the electronegativity difference between the two elements is high and due to the difference in the electronegativity the ionic bond is polar.
The difference in the electronegativity values also tells that the bond between the cation and anion has an ionic character.
Therefore, Magnesium and chlorine will form an ionic bond in magnesium chloride.
Note:
The difference between Electronegativity and Electropositivity –
The ability of an atom to attract a shared pair of bonded electrons is electronegativity. Non-metals have high electronegativities compared to Metals.
The ability of an atom to donate electrons to form positively charged elements is electropositivity. Metallic elements have electropositivity.
Complete answer:
Magnesium belongs to Group-2 i.e Alkaline earth metals whereas chlorine is a non-metal that belongs to Group-7 i.e Halogens. The two elements do not share electrons, So there is no covalent bonding between them.
The magnesium atom gives away two electrons and forms a stable 2+ ion of magnesium whereas the chlorine atom accepts the electron and forms a stable 1- ion of chlorine which results in the formation of an ionic bond.

The electronegativity of two elements are as follows:
Magnesium- 1.31
Chlorine- 3.16
We have to find out the difference in their electronegativities, we can subtract the smaller value of electronegativity from the bigger value of electronegativity.
\[\Delta En=E{{n}_{Cl}}-E{{n}_{Mg}}\]
$\Delta En=3.16-1.31$
$\vartriangle En=1.85$
Here ionic bonding happens because the electronegativity difference between the two elements is high and due to the difference in the electronegativity the ionic bond is polar.
The difference in the electronegativity values also tells that the bond between the cation and anion has an ionic character.
Therefore, Magnesium and chlorine will form an ionic bond in magnesium chloride.
Note:
The difference between Electronegativity and Electropositivity –
The ability of an atom to attract a shared pair of bonded electrons is electronegativity. Non-metals have high electronegativities compared to Metals.
The ability of an atom to donate electrons to form positively charged elements is electropositivity. Metallic elements have electropositivity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




