
What is dipole-dipole attraction and dipole-induced dipole?
Answer
573.6k+ views
Hint: There are few types of forces of attraction or interaction that exist between molecules to hold them together. The type of interaction depends on the polarity of the molecules. Dipole-dipole interaction exists between polar molecules and dipole-induced dipole interaction exists between polar and nonpolar molecules.
Complete Solution :
- In the question it is asked to write about the dipole-dipole attraction and dipole-induced dipole.
Dipole-Dipole attraction:
- It occurs in between polar molecules.
- In polar molecules one atom has high electronegativity and another has less electronegativity. Due to this difference in electronegativity of the atoms the electron cloud is going to distribute between the atoms in polar molecules unequally.
- At this time a partial positive charge is going to create an atom which has less electronegativity and a partial negative charge is going to create an atom which has high electronegativity.
- Now in one polar molecule there are two partial charges (partial positive charge and partial negative charge).
- The partial positive charge in one polar molecule is going to interact with a partial negative charge on another polar molecule.
- This type of interaction is called dipole-dipole attraction.
- The representation of the dipole-dipole interaction in the form of a picture is as follows.

- The examples for dipole-dipole interaction are between Ammonia, sulphur dioxide, Hydrogen fluoride, hydrogen sulphide etc.
Dipole-induced dipole attraction:
- Some polar molecules induce some interaction with nonpolar molecules. This type of interaction is called dipole-induced dipole attraction.
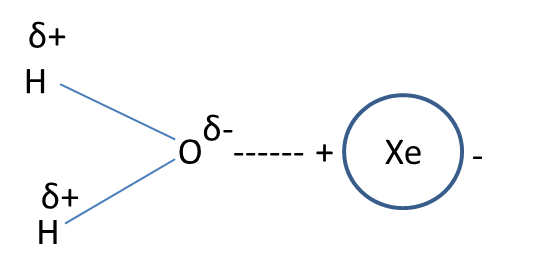
- Examples for this type of interaction are interaction between water (polar) and carbon tetrachloride (nonpolar), interaction between water (polar) and xenon (nonpolar).
- The representation of the dipole-dipole induced interaction in the form of a picture is as follows.
Note: Dipole –dipole interaction occurs in between polar molecules only. A polar molecule is going to induce a dipole in a nonpolar molecule and it is called dipole-dipole induced interaction. Polarity of the molecules is going to depend on the electronegativity of the atoms present in them.
Complete Solution :
- In the question it is asked to write about the dipole-dipole attraction and dipole-induced dipole.
Dipole-Dipole attraction:
- It occurs in between polar molecules.
- In polar molecules one atom has high electronegativity and another has less electronegativity. Due to this difference in electronegativity of the atoms the electron cloud is going to distribute between the atoms in polar molecules unequally.
- At this time a partial positive charge is going to create an atom which has less electronegativity and a partial negative charge is going to create an atom which has high electronegativity.
- Now in one polar molecule there are two partial charges (partial positive charge and partial negative charge).
- The partial positive charge in one polar molecule is going to interact with a partial negative charge on another polar molecule.
- This type of interaction is called dipole-dipole attraction.
- The representation of the dipole-dipole interaction in the form of a picture is as follows.

- The examples for dipole-dipole interaction are between Ammonia, sulphur dioxide, Hydrogen fluoride, hydrogen sulphide etc.
Dipole-induced dipole attraction:
- Some polar molecules induce some interaction with nonpolar molecules. This type of interaction is called dipole-induced dipole attraction.
- Examples for this type of interaction are interaction between water (polar) and carbon tetrachloride (nonpolar), interaction between water (polar) and xenon (nonpolar).
- The representation of the dipole-dipole induced interaction in the form of a picture is as follows.

Note: Dipole –dipole interaction occurs in between polar molecules only. A polar molecule is going to induce a dipole in a nonpolar molecule and it is called dipole-dipole induced interaction. Polarity of the molecules is going to depend on the electronegativity of the atoms present in them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




