
Dissolving sugar in water is a chemical change. True or False?
A. True
B. False
Answer
606.3k+ views
Hint: We should be knowing the basic difference between the physical change and chemical change so as to answer this question. Even we should be familiar with the reaction when sugar is dissolved in water.
Complete step-by-step answer:
Physical changes and changes affecting the form of a chemical substance but not a chemical composition. Physical changes are used to separate mixtures into their component compounds but cannot usually be used to separate compounds into chemical elements or similar compounds.
Chemical changes occur when a substance combines with another way to form a new substance called chemical synthesis or alternatively chemical decomposition into two or more different substances. These processes are called chemical reactions and in general are not reversible except by for the chemical reactions.
Dissolving sugar in water is a physical change because sugar molecules are dispersed within the water but the individual sugar molecules are unchanged.
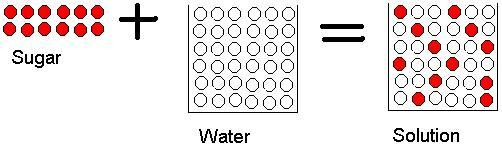
Therefore we can see that dissolving sugar in water is a physical change and the answer to this question is false. So Option B is a correct option.
Note: We should know that in a physical change the molecules are rearranged while their actual composition remains the same. In a chemical change the molecular composition of a substance completely changes and a new system is formed.
Some examples of physical changes: freezing of water, melting of wax, boiling of water etc.
Melting and burning of candle wax is an example of both physical and chemical change. Burning of wood is an example of both physical and chemical change.
Complete step-by-step answer:
Physical changes and changes affecting the form of a chemical substance but not a chemical composition. Physical changes are used to separate mixtures into their component compounds but cannot usually be used to separate compounds into chemical elements or similar compounds.
Chemical changes occur when a substance combines with another way to form a new substance called chemical synthesis or alternatively chemical decomposition into two or more different substances. These processes are called chemical reactions and in general are not reversible except by for the chemical reactions.
Dissolving sugar in water is a physical change because sugar molecules are dispersed within the water but the individual sugar molecules are unchanged.

Therefore we can see that dissolving sugar in water is a physical change and the answer to this question is false. So Option B is a correct option.
Note: We should know that in a physical change the molecules are rearranged while their actual composition remains the same. In a chemical change the molecular composition of a substance completely changes and a new system is formed.
Some examples of physical changes: freezing of water, melting of wax, boiling of water etc.
Melting and burning of candle wax is an example of both physical and chemical change. Burning of wood is an example of both physical and chemical change.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




