
What does a Lewis dot diagram allow you to show?
A. The valence electrons in an atom
B. All of the electrons in an atom
C. The valence protons in an atom
D. The neutrons, protons and electrons in an atom
E. How an atom bonds with other atoms
Answer
531.6k+ views
Hint: Lewis dot structure diagram gives us information about covalent compounds. The total number of valence electrons of any molecule is written as dots on the respective atoms, for a Lewis structure of any molecule.
Complete answer:
Lewis dot structures also called electron dot structures are a way to write the elements along with their valence electrons, made as dots in the representation of any molecule. The Lewis structure of any molecule is represented by the type of bonds that the atoms in that molecule contain; they may be single bonds, double bonds, or triple bonds. Apart from bonds it gives information about lone pairs of electrons by representing dots as electrons on atoms.
For example Lewis structure for carbon dioxide $C{{O}_{2}}$molecule can be written as, $[:\ddot{O}=\ddot{O}:]$
Lewis structure of carbon dioxide is,
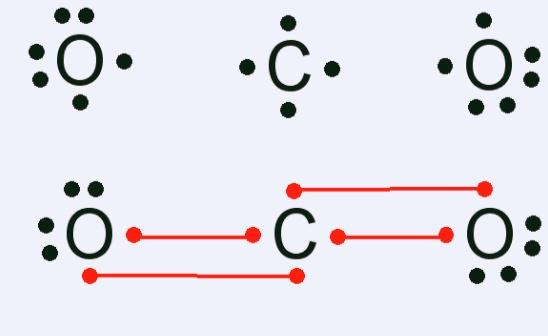
This implies that, in a Lewis structure, total valence electrons are added for combining atoms, (16 valence electrons in carbon dioxide). These electrons are distributed as bond and lone pairs of electrons, (forming 2 double bonds of 8 electrons here). Shared pairs of electrons make single, and then double or triple bonds. Also the least electronegative element occupies the central position.
Hence, the Lewis diagram of any molecules shows the valence electrons in an atom, and how an atom bonds with another atom. So, option A and E are correct.
Note:
For drawing a Lewis structure, for anion, one negative charge means one electron is added, and for cation, one positive charge means one electron is removed. The concept is known as Kossel and Lewis approach of chemical bonds, where Kossel took ionic compounds, while Lewis explained covalent compounds.
Complete answer:
Lewis dot structures also called electron dot structures are a way to write the elements along with their valence electrons, made as dots in the representation of any molecule. The Lewis structure of any molecule is represented by the type of bonds that the atoms in that molecule contain; they may be single bonds, double bonds, or triple bonds. Apart from bonds it gives information about lone pairs of electrons by representing dots as electrons on atoms.
For example Lewis structure for carbon dioxide $C{{O}_{2}}$molecule can be written as, $[:\ddot{O}=\ddot{O}:]$
Lewis structure of carbon dioxide is,

This implies that, in a Lewis structure, total valence electrons are added for combining atoms, (16 valence electrons in carbon dioxide). These electrons are distributed as bond and lone pairs of electrons, (forming 2 double bonds of 8 electrons here). Shared pairs of electrons make single, and then double or triple bonds. Also the least electronegative element occupies the central position.
Hence, the Lewis diagram of any molecules shows the valence electrons in an atom, and how an atom bonds with another atom. So, option A and E are correct.
Note:
For drawing a Lewis structure, for anion, one negative charge means one electron is added, and for cation, one positive charge means one electron is removed. The concept is known as Kossel and Lewis approach of chemical bonds, where Kossel took ionic compounds, while Lewis explained covalent compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




