
Does ammonia have a hydrogen bond?
Answer
510k+ views
Hint: The ammonia is a chemical compound having the formula, \[N{H_3}\] which contains nitrogen as well as hydrogen atoms. And the ammonia is a colorless gas having a pungent smell. This is mainly used as a fertilizer in agricultural areas. According to valence shell electron pair repulsion theory, the ammonia have a trigonal pyramidal shape with bond angle \[106.7^\circ \].
Complete answer:
We need to know that the ammonia molecule has a strong covalent molecule by sharing electrons between nitrogen and hydrogen atoms. And it has total three hydrogen bonding by the acceptance and donation. The lone pair of electrons present in nitrogen is equal to one. Hence, the amount of hydrogen bonding in ammonia is limited. And by using the lone pairs, each ammonia molecule can form one H- bond.
Even though the ammonia is powerfully attacking the hydrogen bonds in gaseous phase. And the stereochemistry of incapacitated activities of \[N{H_3}\] is controlled by using lone pair orbital. And it indicates the characterization of ammonia as a powerful Lewis base. Let’s see the structure of ammonia,
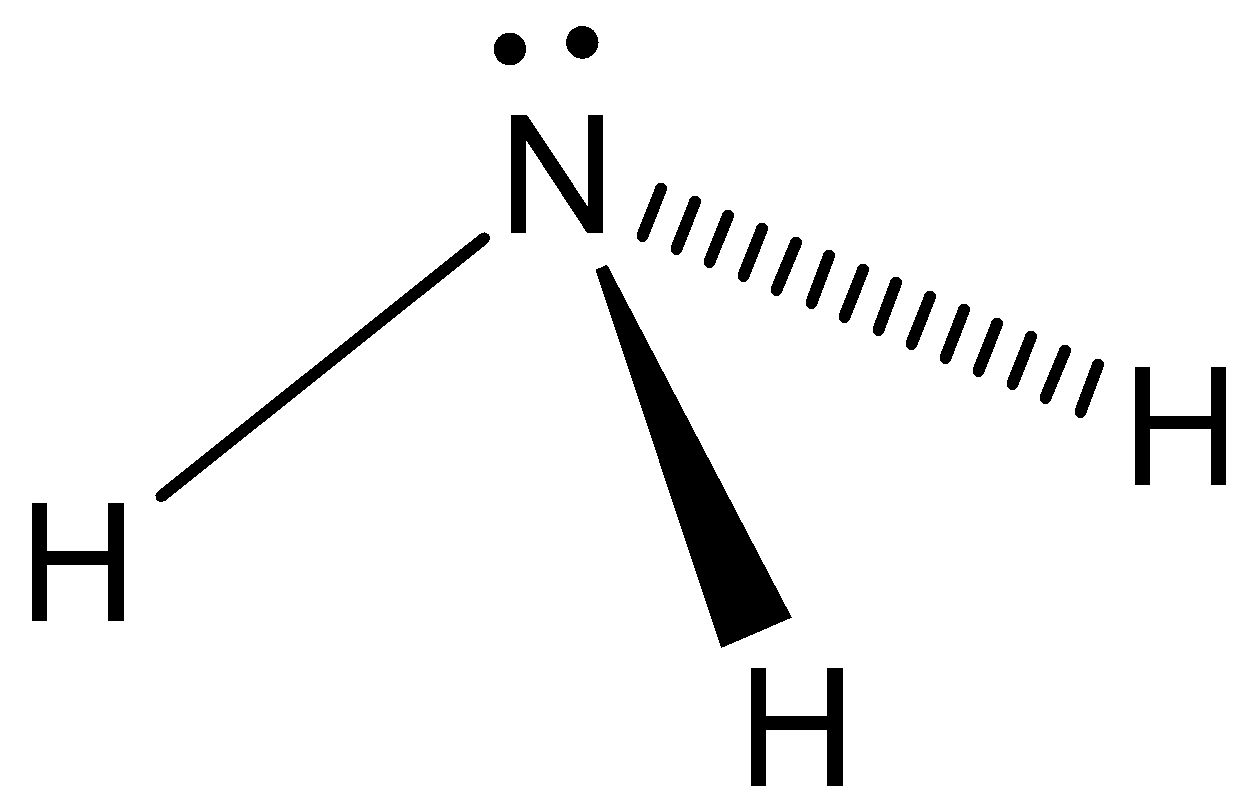
Note:
We need to know that the nitrogen is a highly electronegative atom and the ammonia can form the hydrogen bonding. Because, here the hydrogen is covalently attached with a highly electronegative nitrogen atom. And the size of nitrogen is small. The hydrogen bond is the strongest bond, because it has only one electron. Therefore, there is a strong attraction between strong atoms and weak atoms. And a high amount of energy is required to break that bond. And the hydrogen bonds are mainly of two types, intermolecular $H - $ bonding and intramolecular $H - $ bonding.
Complete answer:
We need to know that the ammonia molecule has a strong covalent molecule by sharing electrons between nitrogen and hydrogen atoms. And it has total three hydrogen bonding by the acceptance and donation. The lone pair of electrons present in nitrogen is equal to one. Hence, the amount of hydrogen bonding in ammonia is limited. And by using the lone pairs, each ammonia molecule can form one H- bond.
Even though the ammonia is powerfully attacking the hydrogen bonds in gaseous phase. And the stereochemistry of incapacitated activities of \[N{H_3}\] is controlled by using lone pair orbital. And it indicates the characterization of ammonia as a powerful Lewis base. Let’s see the structure of ammonia,

Note:
We need to know that the nitrogen is a highly electronegative atom and the ammonia can form the hydrogen bonding. Because, here the hydrogen is covalently attached with a highly electronegative nitrogen atom. And the size of nitrogen is small. The hydrogen bond is the strongest bond, because it has only one electron. Therefore, there is a strong attraction between strong atoms and weak atoms. And a high amount of energy is required to break that bond. And the hydrogen bonds are mainly of two types, intermolecular $H - $ bonding and intramolecular $H - $ bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




