
Does \[LiAl{H_4}\] reduce Amides?
Answer
507k+ views
Hint: \[LiAl{H_4}\] is a very strong reducing agent. It reduces aldehydes, amides, ketones, esters, carboxylic acid and even carboxylate salts to alcohols.
Amide is a functional group that contains both nitrogen and carboxyl groups in it. It is usually represented as below:
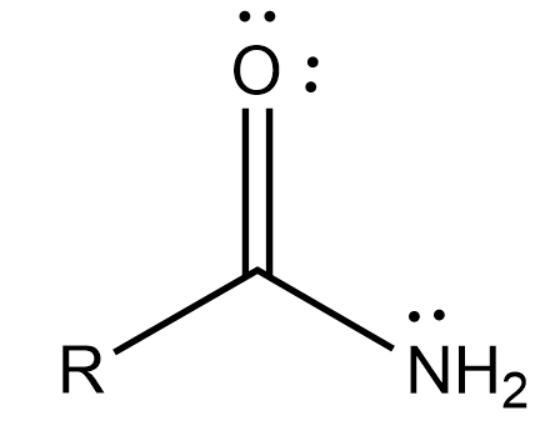
\[LiAl{H_4}\] will reduce Amides to Amines and this means that the oxygen that was present in the amide is removed from the molecule after the reaction completes.
Complete answer:
Let’s see how the reaction occurs when amide is treated with \[LiAl{H_4}\]
First there is a nucleophilic attack by one of the hydrides of \[LiAl{H_4}\] . Because the carbonyl group is highly polar the oxygen becomes negative and thus \[Al\] having negatively charge can now attack the positively charged carbonyl carbon and thus the following step occurs
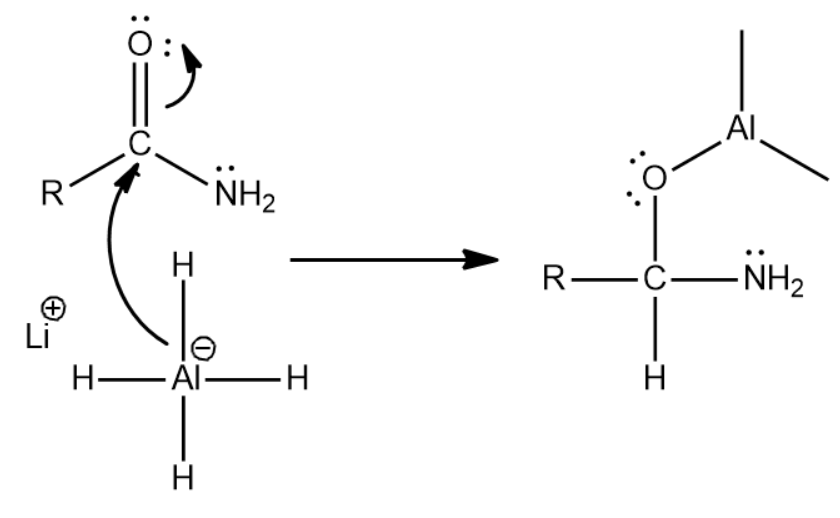
Now since there is an oxygen that can act as a leaving group we will get the following reaction which leads to the formation of an iminium ion
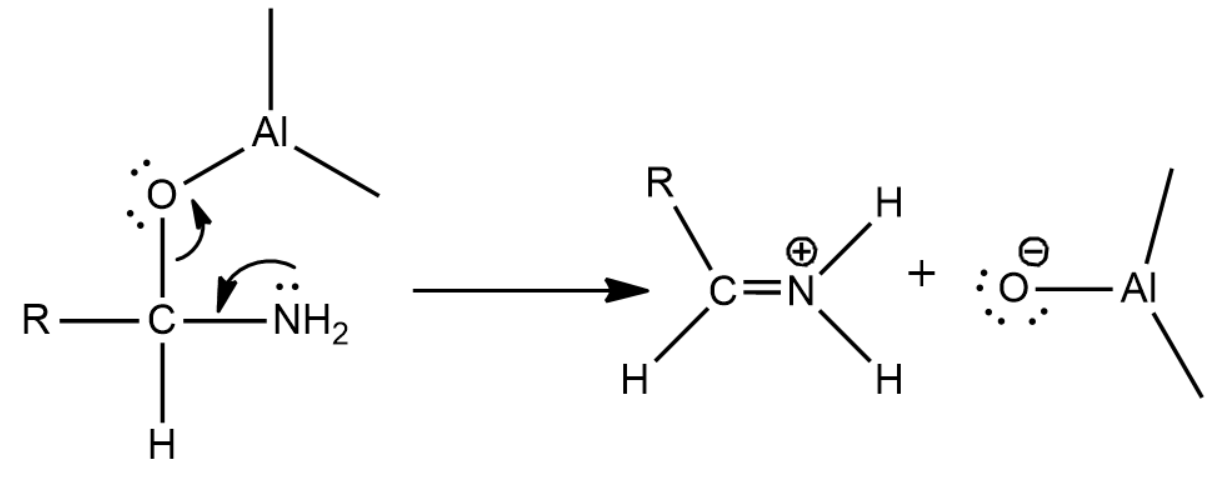
Now there is a nucleophilic attack by the hydride of \[LiAl{H_4}\] causing for the formation of the final product, amine
That is given by the following reaction:
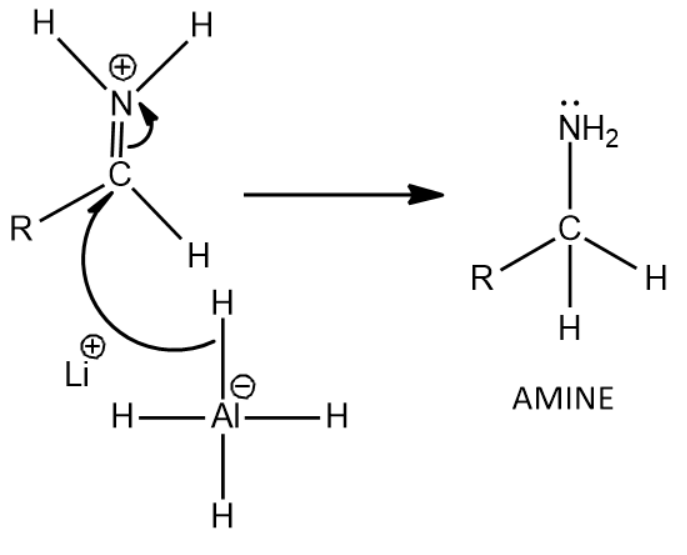
Thus we get Amine as the product when amides are treated with \[LiAl{H_4}\] which is a strong reducing agent.
Thus we can write the general form of the reaction as:

Note:
\[NaB{H_4}\] is also a reducing agent but it is very less reactive than \[LiAl{H_4}\]. \[NaB{H_4}\] can reduce aldehydes, ketones and acid chlorides but cannot reduce amides or esters.
Amides can be converted to \[{1^o},{\text{ }}{2^o}{\text{ or }}{3^o}\]amine just by using \[LiAl{H_4}\]. For doing this we have to select the suitable amides to begin the reaction with.
The purpose of using \[{H_2}O\] at the end is for a bit of workup. It neutralizes strongly basic reagents at the end of the reaction thus helping in the rate of the reaction.
Amide is a functional group that contains both nitrogen and carboxyl groups in it. It is usually represented as below:

\[LiAl{H_4}\] will reduce Amides to Amines and this means that the oxygen that was present in the amide is removed from the molecule after the reaction completes.
Complete answer:
Let’s see how the reaction occurs when amide is treated with \[LiAl{H_4}\]
First there is a nucleophilic attack by one of the hydrides of \[LiAl{H_4}\] . Because the carbonyl group is highly polar the oxygen becomes negative and thus \[Al\] having negatively charge can now attack the positively charged carbonyl carbon and thus the following step occurs

Now since there is an oxygen that can act as a leaving group we will get the following reaction which leads to the formation of an iminium ion

Now there is a nucleophilic attack by the hydride of \[LiAl{H_4}\] causing for the formation of the final product, amine
That is given by the following reaction:

Thus we get Amine as the product when amides are treated with \[LiAl{H_4}\] which is a strong reducing agent.
Thus we can write the general form of the reaction as:

Note:
\[NaB{H_4}\] is also a reducing agent but it is very less reactive than \[LiAl{H_4}\]. \[NaB{H_4}\] can reduce aldehydes, ketones and acid chlorides but cannot reduce amides or esters.
Amides can be converted to \[{1^o},{\text{ }}{2^o}{\text{ or }}{3^o}\]amine just by using \[LiAl{H_4}\]. For doing this we have to select the suitable amides to begin the reaction with.
The purpose of using \[{H_2}O\] at the end is for a bit of workup. It neutralizes strongly basic reagents at the end of the reaction thus helping in the rate of the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




