
Does nitroethane show tautomerism?
Answer
535.2k+ views
Hint: The phenomenon in which different compounds having the same chemical formula but they have different structures is isomerism, and those compounds are known as isomers, and this is an important phenomenon for compounds. Some types of isomerism are Positional isomerism, chain isomerism, tautomerism, functional isomerism, etc.
Complete step by step answer: The isomers in functional differ with respect to the nature of the functional groups and hence belong to different families.
- Tautomers are actually functional isomers. They exist simultaneously and also in dynamic equilibrium. There are many types of tautomerism and keto-enol tautomerism is very common among them.
- This tautomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,
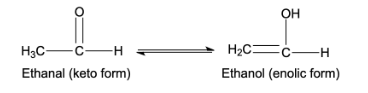
- If the compound has alpha-hydrogen atom in the compound, only then the compound can show the keto-enol tautomerism because it is a necessary condition.
- Now for the question, the structure of nitroethane is
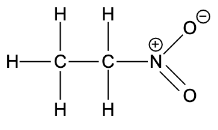
- As we can clearly see the presence of. alpha hydrogen in the structure shown above. Thus, nitroethane shows tautomerism.
Additional information: As per the rule of keto-enol tautomerism, the hydroxy; group in all alcohols is attached directly with a double-bonded carbon atom, and forms aldehydes or ketones.
Note: It is important to note that an aldehyde or ketone shows tautomerism only when there is a presence of alpha hydrogen. The alpha hydrogen involved in 1,3 migration. Another example of a compound that doesn’t show tautomerism is benzaldehyde.
Complete step by step answer: The isomers in functional differ with respect to the nature of the functional groups and hence belong to different families.
- Tautomers are actually functional isomers. They exist simultaneously and also in dynamic equilibrium. There are many types of tautomerism and keto-enol tautomerism is very common among them.
- This tautomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,

- If the compound has alpha-hydrogen atom in the compound, only then the compound can show the keto-enol tautomerism because it is a necessary condition.
- Now for the question, the structure of nitroethane is

- As we can clearly see the presence of. alpha hydrogen in the structure shown above. Thus, nitroethane shows tautomerism.
Additional information: As per the rule of keto-enol tautomerism, the hydroxy; group in all alcohols is attached directly with a double-bonded carbon atom, and forms aldehydes or ketones.
Note: It is important to note that an aldehyde or ketone shows tautomerism only when there is a presence of alpha hydrogen. The alpha hydrogen involved in 1,3 migration. Another example of a compound that doesn’t show tautomerism is benzaldehyde.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




