
What does phenol form by reaction with $HCN$ and $HCl$.
Answer
505.8k+ views
Hint: Phenol is an aromatic compound in which electrons are delocalized. When phenol is reacted with the mixture of $HCN$and $HCl$ it forms aldimine as the intermediate. The catalyst we use for this reaction is $ZnC{l_2}$. Thus we do hydrolysis of the intermediate product to get the final product.
Complete answer:
Phenol is an aromatic compound which contains hydroxyl groups. The mixture of $HCN$and $HCl$is reacted with phenol in the presence of a catalyst known as zinc chloride. Further an intermediate product is formed which on hydrolysis yields us $p - $hydroxybenzaldehyde. The whole reaction will take place in the following steps:
$1.$In the first stage of reaction, the mixture of hydrochloric acid and hydrogen cyanide is reacted to form a complex in the presence of the zinc chloride.
$HCl{\text{ + HCN }}\xrightarrow{{ZnC{l_2}}}{\text{ Cl - CH = NH}}$
The formed complex will further react with the phenol and form an intermediate product.
$2.$ Now the given complex will react with phenol and produce an intermediate product known as aldimine. Then the aldimine so formed will undergo hydrolysis.
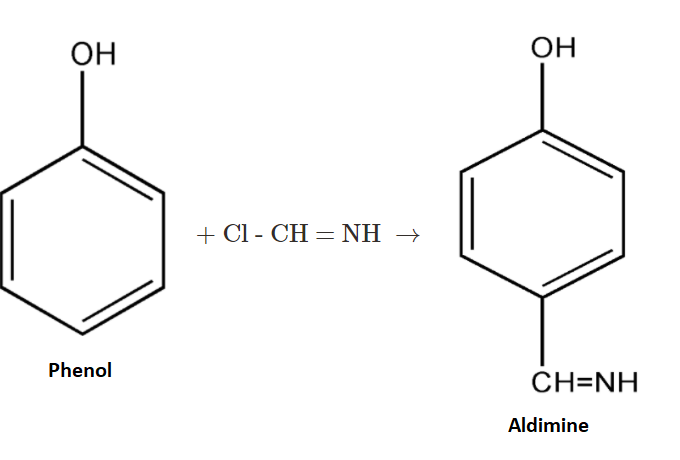
$3.$Now the formed intermediate, Aldimine, is further undergoing hydrolysis with ${H_3}{O^ + }$. On hydrolysis of the above intermediate we get $p - $hydroxybenzaldehyde as our main product.
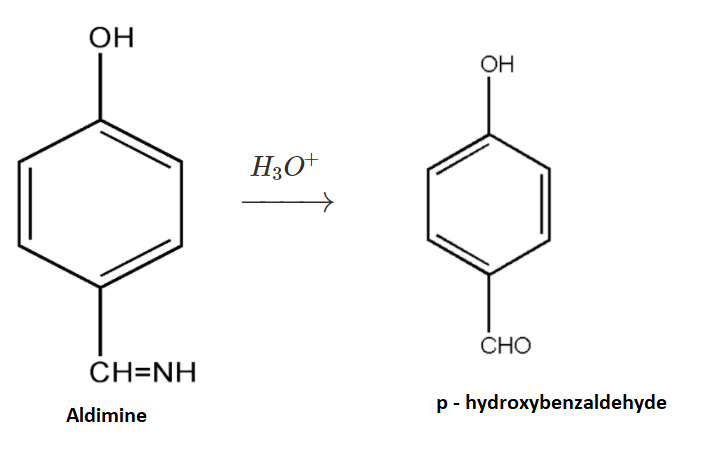
The product so formed is $p - $hydroxybenzaldehyde. The reaction is also known as the Gattermann Reaction used for synthesis of aromatic aldehydes.
Note:
The intermediate formed during the reaction is a result of zinc chloride. Here zinc chloride acts as a catalyst. We can use others like Aluminium Chloride in place of Zinc Chloride. On hydrolysis there is addition of oxygen and an amine group if formed as a by-product.
Complete answer:
Phenol is an aromatic compound which contains hydroxyl groups. The mixture of $HCN$and $HCl$is reacted with phenol in the presence of a catalyst known as zinc chloride. Further an intermediate product is formed which on hydrolysis yields us $p - $hydroxybenzaldehyde. The whole reaction will take place in the following steps:
$1.$In the first stage of reaction, the mixture of hydrochloric acid and hydrogen cyanide is reacted to form a complex in the presence of the zinc chloride.
$HCl{\text{ + HCN }}\xrightarrow{{ZnC{l_2}}}{\text{ Cl - CH = NH}}$
The formed complex will further react with the phenol and form an intermediate product.
$2.$ Now the given complex will react with phenol and produce an intermediate product known as aldimine. Then the aldimine so formed will undergo hydrolysis.

$3.$Now the formed intermediate, Aldimine, is further undergoing hydrolysis with ${H_3}{O^ + }$. On hydrolysis of the above intermediate we get $p - $hydroxybenzaldehyde as our main product.

The product so formed is $p - $hydroxybenzaldehyde. The reaction is also known as the Gattermann Reaction used for synthesis of aromatic aldehydes.
Note:
The intermediate formed during the reaction is a result of zinc chloride. Here zinc chloride acts as a catalyst. We can use others like Aluminium Chloride in place of Zinc Chloride. On hydrolysis there is addition of oxygen and an amine group if formed as a by-product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




