
What does the following symbol stand for?
$ {H_2} $
Answer
507.9k+ views
Hint :Recall that the symbol $ H $ stands for hydrogen in the periodic table. Indicating 2 as subscript will mean that the atoms of hydrogen are combining to form a diatomic molecule. Every time we use a subscript it means that there is more than one atom of the same element present in our molecule.
Complete Step By Step Answer:
We know that in the periodic table the first element is hydrogen which is represented by the letter ‘ $ H $ ’. The word hydrogen is derived from the Greek words “hydro” and “genes” meaning water forming.
Hydrogen atom can form a covalent bond with another hydrogen atom forming a diatomic molecule called dihydrogen gas. This is represented as $ {H_2} $ .
The dihydrogen is the simplest covalent bond in which one of the hydrogen shares its sole electron with another hydrogen thus attaining a stable electronic configuration (Duet configuration). The molecule has a linear shape and is non polar in nature.
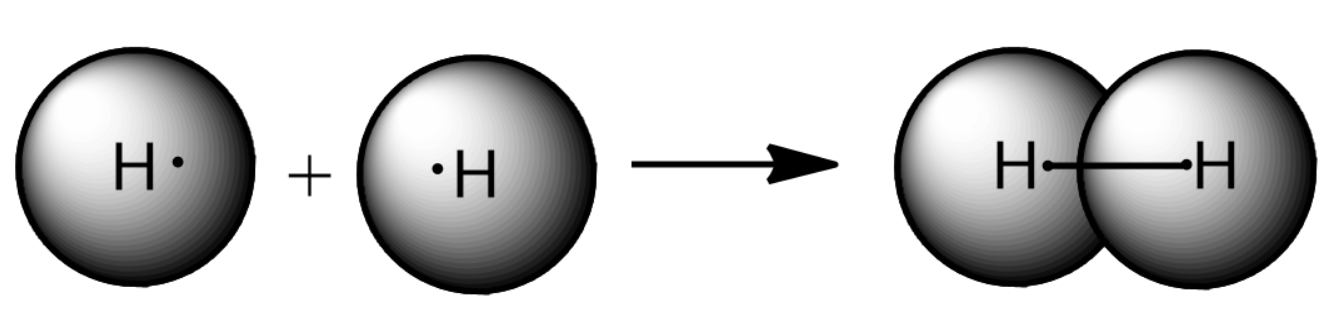
Represented above is the formation of a dihydrogen bond by the overlap of the s-orbital of Hydrogen. This will form a sigma bond and thus di hydrogen is formed. This Dihydrogen is represented as $ {H_2} $
If we say this in terms of molecular orbital theory then we can say that the atomic orbital of each of the hydrogen atoms combine together to form a molecular orbital. The electrons occupy the molecular orbital of lowest energy, the $ {\sigma _{1s}}\; $ molecular bonding orbital.
Note :
Apart from Hydrogen there are many other molecules that can be represented by using 2 as a subscript. $ {O_2} $ , $ {N_2} $ , $ C{l_2} $ are some examples of such homonuclear diatomic molecules.
Isotopes of hydrogen can also exist as diatomic molecules. Deuterium is an isotope of hydrogen and its diatomic form is called heavy hydrogen and is represented as $ {D_2} $ . Deuterium is a highly flammable and asphyxiant gas.
Complete Step By Step Answer:
We know that in the periodic table the first element is hydrogen which is represented by the letter ‘ $ H $ ’. The word hydrogen is derived from the Greek words “hydro” and “genes” meaning water forming.
Hydrogen atom can form a covalent bond with another hydrogen atom forming a diatomic molecule called dihydrogen gas. This is represented as $ {H_2} $ .
The dihydrogen is the simplest covalent bond in which one of the hydrogen shares its sole electron with another hydrogen thus attaining a stable electronic configuration (Duet configuration). The molecule has a linear shape and is non polar in nature.

Represented above is the formation of a dihydrogen bond by the overlap of the s-orbital of Hydrogen. This will form a sigma bond and thus di hydrogen is formed. This Dihydrogen is represented as $ {H_2} $
If we say this in terms of molecular orbital theory then we can say that the atomic orbital of each of the hydrogen atoms combine together to form a molecular orbital. The electrons occupy the molecular orbital of lowest energy, the $ {\sigma _{1s}}\; $ molecular bonding orbital.
Note :
Apart from Hydrogen there are many other molecules that can be represented by using 2 as a subscript. $ {O_2} $ , $ {N_2} $ , $ C{l_2} $ are some examples of such homonuclear diatomic molecules.
Isotopes of hydrogen can also exist as diatomic molecules. Deuterium is an isotope of hydrogen and its diatomic form is called heavy hydrogen and is represented as $ {D_2} $ . Deuterium is a highly flammable and asphyxiant gas.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




