
How does the metal reactivity series work?
Answer
564.3k+ views
Hint Metals are those substances which have shiny surface, shows the property of malleability and ductility, generally solid at room temperature, produces sound when hit known as sonorous property and usually good conductor of heat and electricity.
Complete answer:
Reactivity series is the series of metals which arrange the metal on their reactivity bases from highest reactive metal to least reactive one. It is also known by the name activity series. Metals show reactivity because of their incomplete outer orbitals i.e. presence of valence electrons or due to their electronic configuration. Metals form positively charged ions known as cations when they tend to lose electrons and when they gain electrons they form negatively charged ions known as anions.
-Metals from potassium to calcium are highly reactive even it can react with water too, metals from magnesium to lead can react with acids and metals from copper to platinum are highly unreactive that they don’t react with any other substance in normal conditions that’s why these metals will not easily corrode. Reactivity series can be shown as:
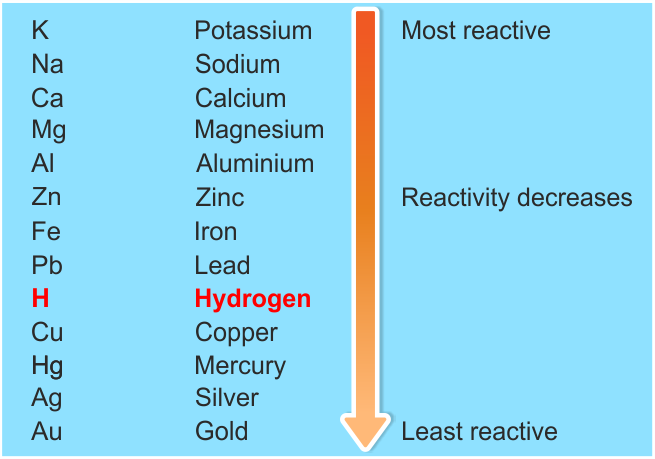
-Hydrogen is non-metal but still is in the reactivity series as it helps in the comparison of metals reactivity.
-Hence reactivity series of metal describe which metal is more reactive and which one is least reactive.
Note: Metals who have a high atomic number tend to be more reactive as their electrons are far away from the nucleus i.e. less tightly bound with nucleus so they can be removed easily as compared to nearer one which are tightly bound with nucleus.
Complete answer:
Reactivity series is the series of metals which arrange the metal on their reactivity bases from highest reactive metal to least reactive one. It is also known by the name activity series. Metals show reactivity because of their incomplete outer orbitals i.e. presence of valence electrons or due to their electronic configuration. Metals form positively charged ions known as cations when they tend to lose electrons and when they gain electrons they form negatively charged ions known as anions.
-Metals from potassium to calcium are highly reactive even it can react with water too, metals from magnesium to lead can react with acids and metals from copper to platinum are highly unreactive that they don’t react with any other substance in normal conditions that’s why these metals will not easily corrode. Reactivity series can be shown as:

-Hydrogen is non-metal but still is in the reactivity series as it helps in the comparison of metals reactivity.
-Hence reactivity series of metal describe which metal is more reactive and which one is least reactive.
Note: Metals who have a high atomic number tend to be more reactive as their electrons are far away from the nucleus i.e. less tightly bound with nucleus so they can be removed easily as compared to nearer one which are tightly bound with nucleus.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




