
Why does water have a higher boiling point than alcohol? At what temperature in kelvin, it can be changed into solid state and into vapors?
Answer
589.5k+ views
Hint: Water is a chemical compound with the formula of \[{{\text{H}}_{\text{2}}}{\text{O}}\]. In this formula the ratio of hydrogen and oxygen is \[{\text{2:1}}\]. Water can exist as liquid, solid, gas. When water get heated up to boiling it converted in to vapor from liquid and at \[{{\text{0}}^{\text{o}}}{\text{C}}\] water get converted into ice.
Complete step by step answer:
Alcohol is an organic compound in which the hydroxyl group is bound with a saturated carbon atom. Due to the presence of hydroxyl group alcohols can form intermolecular hydrogen bonding. This results in an increase of effective molecular mass. Therefore, the heat required to boil alcohols is greater than that of its corresponding hydrocarbon. The hydrogen bonding in alcohols shown below,
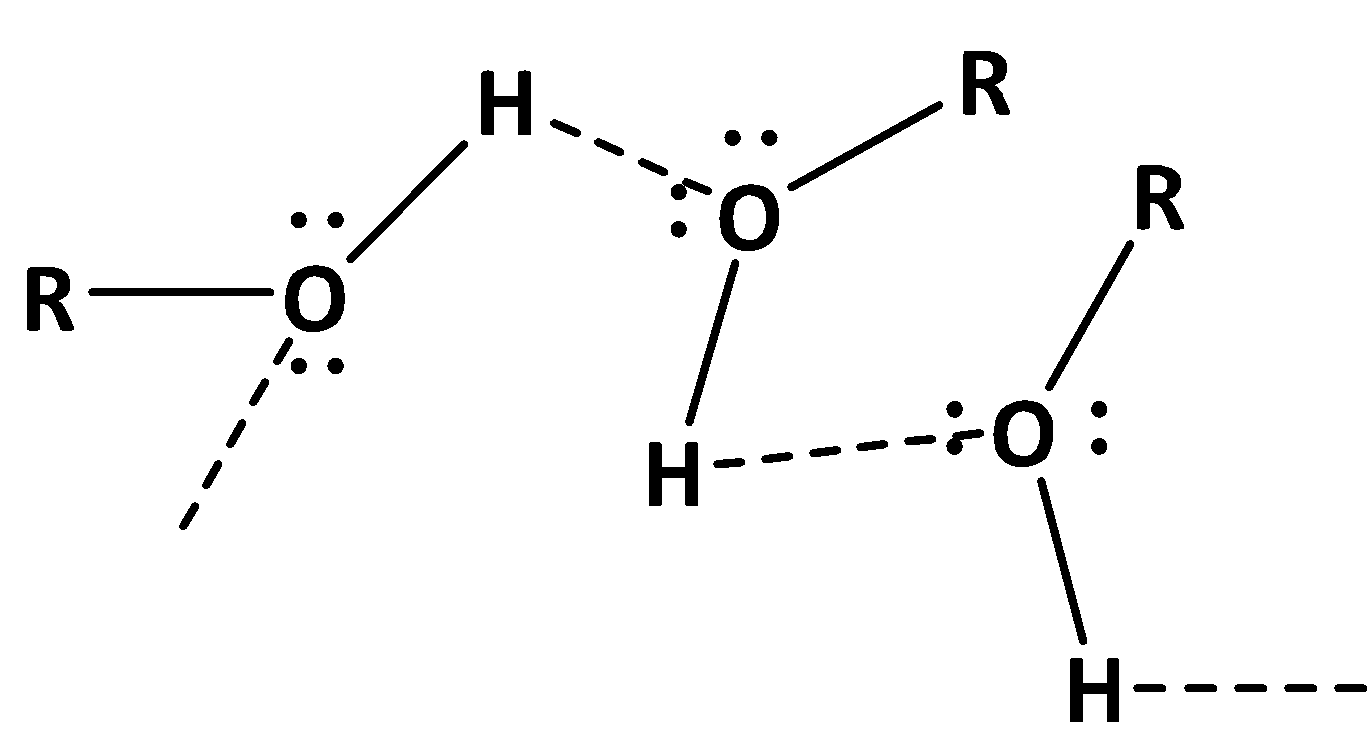
On the other hand, water can form hydrogen bonds too. But due to the presence of two hydrogen atoms attached with the oxygen of water it can form hydrogen bonds greater in number than alcohols. As a result the temperature of boiling of water is also greater in case of water than that of alcohols. The hydrogen bonding in water shown below
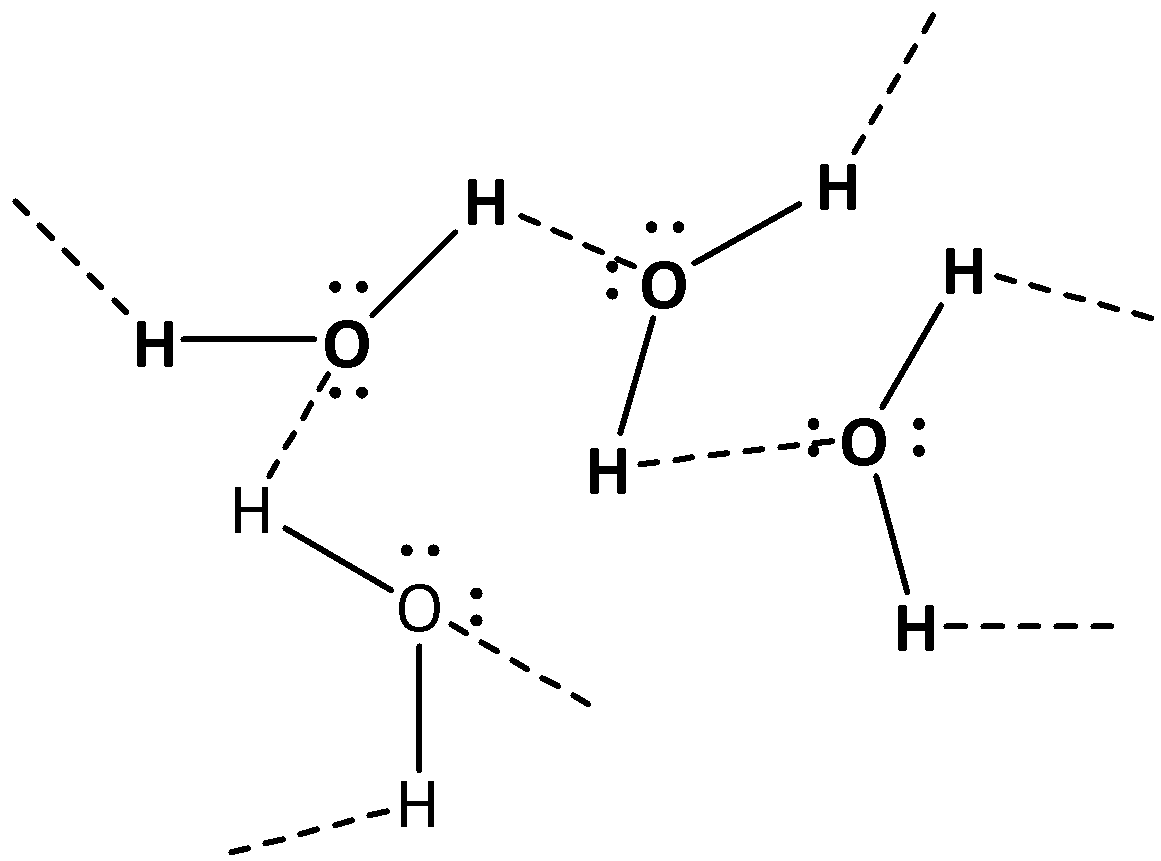
At the temperature of \[{\text{273K}}\] water can be changed into solid state and at \[{\text{373K}}\] water can be changed into vapors.
Note:
The maximum density of water is \[{\text{1gm/c}}{{\text{m}}^3}\]at \[{4^{\text{o}}}{\text{C}}\]. When the temperature is either lower or higher than \[{4^{\text{o}}}{\text{C}}\] the density of the water changes from\[{\text{1gm/c}}{{\text{m}}^3}\]and becomes lower. Among alcohols phenol shows acidic nature, as its corresponding conjugate base get stability by conjugation of the negative charge with the benzene ring.
Complete step by step answer:
Alcohol is an organic compound in which the hydroxyl group is bound with a saturated carbon atom. Due to the presence of hydroxyl group alcohols can form intermolecular hydrogen bonding. This results in an increase of effective molecular mass. Therefore, the heat required to boil alcohols is greater than that of its corresponding hydrocarbon. The hydrogen bonding in alcohols shown below,

On the other hand, water can form hydrogen bonds too. But due to the presence of two hydrogen atoms attached with the oxygen of water it can form hydrogen bonds greater in number than alcohols. As a result the temperature of boiling of water is also greater in case of water than that of alcohols. The hydrogen bonding in water shown below

At the temperature of \[{\text{273K}}\] water can be changed into solid state and at \[{\text{373K}}\] water can be changed into vapors.
Note:
The maximum density of water is \[{\text{1gm/c}}{{\text{m}}^3}\]at \[{4^{\text{o}}}{\text{C}}\]. When the temperature is either lower or higher than \[{4^{\text{o}}}{\text{C}}\] the density of the water changes from\[{\text{1gm/c}}{{\text{m}}^3}\]and becomes lower. Among alcohols phenol shows acidic nature, as its corresponding conjugate base get stability by conjugation of the negative charge with the benzene ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




