
Why does water have different properties than its constituent elements?
Answer
493.2k+ views
Hint: Although the molecules of water are simple in structure ( \[{H_2}O\]), the physical and chemical properties of the compound are extraordinarily complicated, and they are not typical of most substances found on Earth. Water is a tasteless and odorless liquid at room temperature; it has the important ability to dissolve many other substances.
Complete answer:
We can draw the structure of water as,
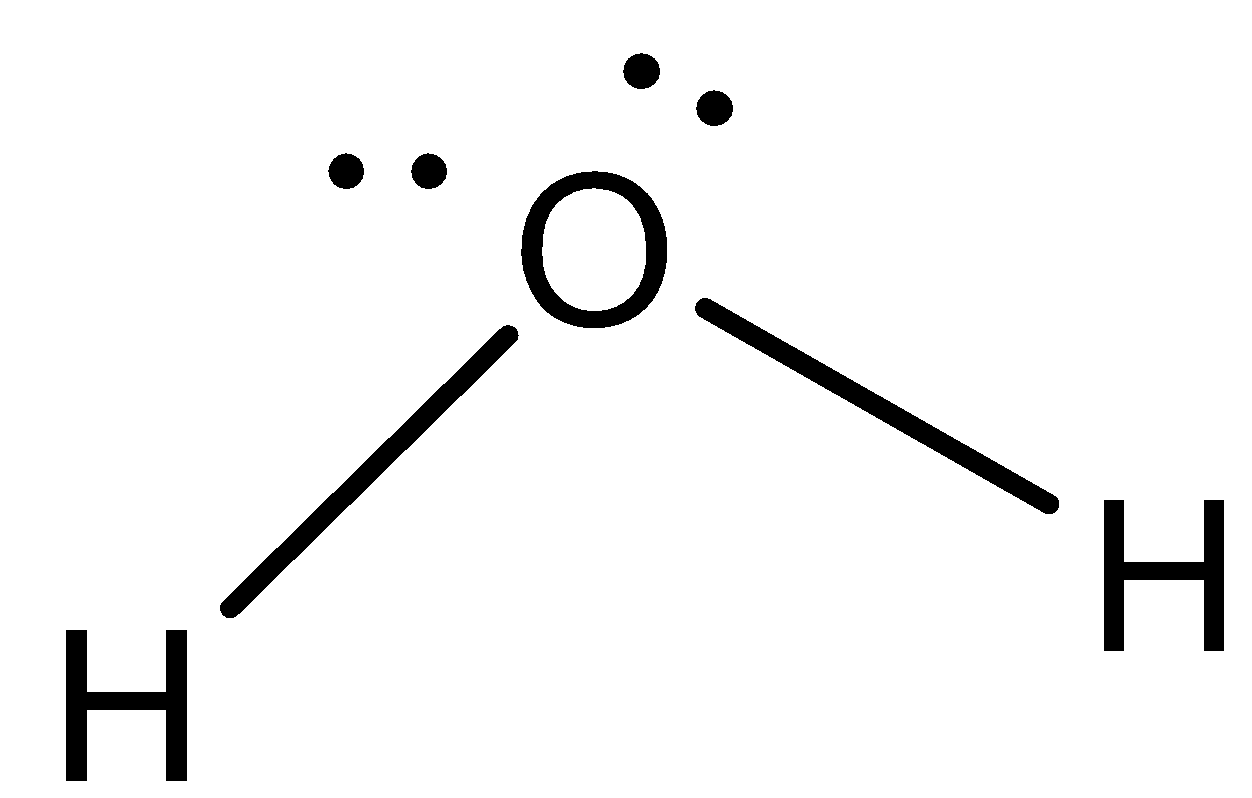
The reaction of water can be represented as the synthesis reaction of water by:
\[{H_2}(g) + \dfrac{1}{2}{O_2}(g) \to {H_2}O(l) + \Delta \]
Now this is a redox reaction in that hydrogen has been oxidized, and oxygen has been reduced to form a very stable molecule. And thus the molecular properties are distinct from the properties of the elemental reactants.
And moreover, to heat the \[H - O\] bonds we have to supply much energy, and this tends to quench the energy supplied by a fire when we douse it. This is especially true given that water, for such a small molecule, is exceptionally dense, and is not even a gas under normal conditions.
As we know, water is a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds
Note:
We know that the constituent elements of water are hydrogen and oxygen. Hydrogen is an inflammable substance, i.e. it burns whereas oxygen supports burning. These properties are completely different from that of water, which extinguishes fire. For example, although the sight of ice cubes floating in a glass of ice water is commonplace, such behavior is unusual for chemical entities. For almost every other compound, the solid state is denser than the liquid state; thus, the solid would sink to the bottom of the liquid.
Complete answer:
We can draw the structure of water as,

The reaction of water can be represented as the synthesis reaction of water by:
\[{H_2}(g) + \dfrac{1}{2}{O_2}(g) \to {H_2}O(l) + \Delta \]
Now this is a redox reaction in that hydrogen has been oxidized, and oxygen has been reduced to form a very stable molecule. And thus the molecular properties are distinct from the properties of the elemental reactants.
And moreover, to heat the \[H - O\] bonds we have to supply much energy, and this tends to quench the energy supplied by a fire when we douse it. This is especially true given that water, for such a small molecule, is exceptionally dense, and is not even a gas under normal conditions.
As we know, water is a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds
Note:
We know that the constituent elements of water are hydrogen and oxygen. Hydrogen is an inflammable substance, i.e. it burns whereas oxygen supports burning. These properties are completely different from that of water, which extinguishes fire. For example, although the sight of ice cubes floating in a glass of ice water is commonplace, such behavior is unusual for chemical entities. For almost every other compound, the solid state is denser than the liquid state; thus, the solid would sink to the bottom of the liquid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




