
Draw a dot structure of \[B{r_3}^{ - 1}\]. Deduce approximate value of bond angle.
Answer
517.5k+ views
Hint: Bromine is from group7 or 17, so it has valence electron seven. We have been given three bromines in question and then there is an additional negative sign indicating additional charge on the structure. So, in total we have three bromines with charge on each is 7.
Complete answer:
The Lewis dot structure is-
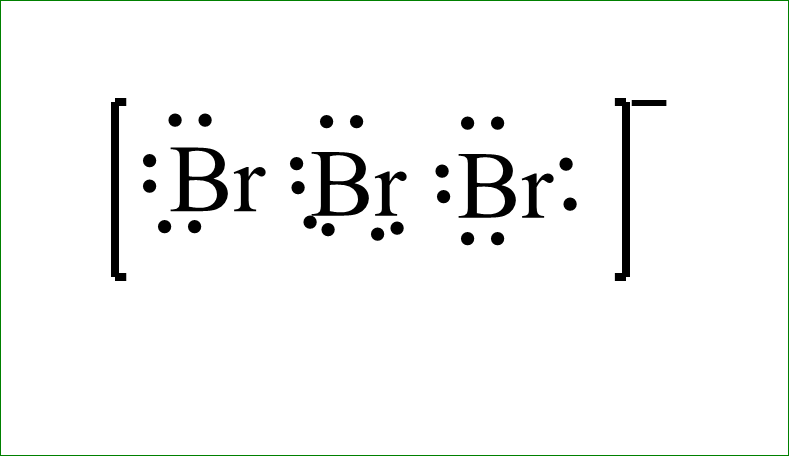
The bond angle is \[{180^ \circ }\], since it is linear in shape. Which makes it twenty-one and additional one negative charge makes it twenty-two electrons. We will line them up in such a way that they get symmetry. We will take the electrons we have and we will place the two electrons between atoms to form a chemical bond. Then we will draw the electrons outside of atoms to fill their octets. We have about 2,4,6,8,16 and then back to the central bromine.,18 and 20. So everything has octets now, but we only use 20. We have additional two valence electrons left which we will place somewhere.
Since bromine is below period second, in the periodic table, that means, it can have an expanded octet. It can have more than eight electrons. So, we will put these last two valence electrons on the central bromine. Now we have used all the twenty-two valence electrons.
It is lowest for fluorine. Hence, the most powerful oxidising agent is fluorine.
Note:
The inner bromine has ten valence electrons but that is okay, because bromine can have expanded octet. The lewis dot structure will be put inside the brackets and the negative sign will be indicated outside it.
Complete answer:
The Lewis dot structure is-
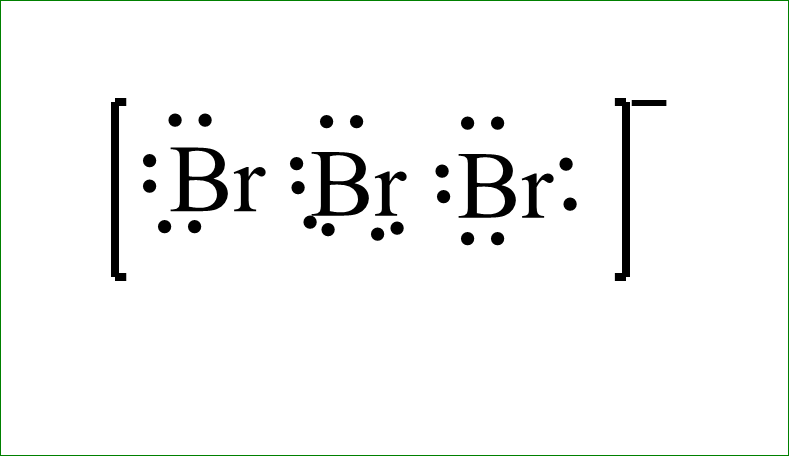
The bond angle is \[{180^ \circ }\], since it is linear in shape. Which makes it twenty-one and additional one negative charge makes it twenty-two electrons. We will line them up in such a way that they get symmetry. We will take the electrons we have and we will place the two electrons between atoms to form a chemical bond. Then we will draw the electrons outside of atoms to fill their octets. We have about 2,4,6,8,16 and then back to the central bromine.,18 and 20. So everything has octets now, but we only use 20. We have additional two valence electrons left which we will place somewhere.
Since bromine is below period second, in the periodic table, that means, it can have an expanded octet. It can have more than eight electrons. So, we will put these last two valence electrons on the central bromine. Now we have used all the twenty-two valence electrons.
It is lowest for fluorine. Hence, the most powerful oxidising agent is fluorine.
Note:
The inner bromine has ten valence electrons but that is okay, because bromine can have expanded octet. The lewis dot structure will be put inside the brackets and the negative sign will be indicated outside it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




