
How can I draw a Lewis dot diagram for carbon dioxide?
Answer
559.2k+ views
Hint: The atomic number of carbon is 6 and the atomic number of oxygen is 8.
- The oxygen can form two single bonds and one double bond with other atoms.
Complete step by step answer:
So in the question it is asked that how, one can draw the Lewis dot diagram of carbon dioxide.
The molecular formula of carbon dioxide is $C{{O}_{2}}$.
- The Lewis dot diagram is also called the Lewis electron dot structure, here the valence electrons present in the compound are represented as dots around the atoms.
To draw the Lewis dot diagram there are mainly five steps that should be followed and let’s discuss the rules briefly through solving this question.
-First find the number of valence electrons present in each atom of the compound and then add up the valence electrons to get the total number of valence electrons in the compound.
- From the lower class we are studying to calculate the valence electrons of the element ,write its electronic configuration or the shell configuration,the electrons present on the outermost shell of the atom are called the valence electrons.
- The atoms present in carbon dioxide are two oxygen atoms and a carbon atom.
Since the atomic number of C is 6 its shell configuration is $\text{K=2,L=4}$ and has 4 valence electrons.
The shell configuration of O is $\text{K=2, L=6}$, since the atomic number is 8 and the valence electrons are 6.
The total valence electron in $C{{O}_{2}}=4 + 2(6) = 16{{e}^{-}}s$
-Next if there is a hydrogen atom present in the compound it should always be placed as the outside atoms i.e. the atoms surrounding the central atom.
Since there is no H in the compound let's skip this step.
-Now find the least electronegative atom in the compound and place it as the central atom.
Here C is the least electronegative than O, as the C is far away from F than O, so C is taken as the central atom and the O is distributed as the outside atoms.
-Now fill two electrons in between the two atoms to show the chemical bonding with the atoms.
-Now complete the octet for the outside atoms i.e. give eight electrons for the outside atoms.
From the $16{{e}^{-}}s$, give the two O eight electrons each so that it completes its octet configuration.
-And now check the octet configuration for all the atoms in the compound if the central atom lacks the octet configuration,then shift the electron pair towards the central atom to form multiple bonds.
Here if we see the distribution of electrons as said earlier, the C lacks electrons.And hence transfer the electron pairs from the outside atom towards the central atom,so that all the electrons will achieve the octet configuration.
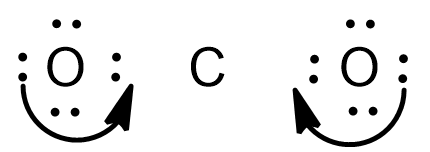
And the final Lewis dot structure of $C{{O}_{2}}$ is as follows:
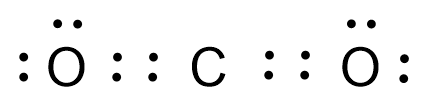
In this structure all the atoms have eight electrons, hence the criteria of octet configuration is satisfied.
Note: The Lewis dot structure gives an idea about the bond angle and about the structure of the compound.Here the structure of carbon dioxide is linear and the O-C-O bond angle is ${{180}^{\circ }}$
- The oxygen can form two single bonds and one double bond with other atoms.
Complete step by step answer:
So in the question it is asked that how, one can draw the Lewis dot diagram of carbon dioxide.
The molecular formula of carbon dioxide is $C{{O}_{2}}$.
- The Lewis dot diagram is also called the Lewis electron dot structure, here the valence electrons present in the compound are represented as dots around the atoms.
To draw the Lewis dot diagram there are mainly five steps that should be followed and let’s discuss the rules briefly through solving this question.
-First find the number of valence electrons present in each atom of the compound and then add up the valence electrons to get the total number of valence electrons in the compound.
- From the lower class we are studying to calculate the valence electrons of the element ,write its electronic configuration or the shell configuration,the electrons present on the outermost shell of the atom are called the valence electrons.
- The atoms present in carbon dioxide are two oxygen atoms and a carbon atom.
Since the atomic number of C is 6 its shell configuration is $\text{K=2,L=4}$ and has 4 valence electrons.
The shell configuration of O is $\text{K=2, L=6}$, since the atomic number is 8 and the valence electrons are 6.
The total valence electron in $C{{O}_{2}}=4 + 2(6) = 16{{e}^{-}}s$
-Next if there is a hydrogen atom present in the compound it should always be placed as the outside atoms i.e. the atoms surrounding the central atom.
Since there is no H in the compound let's skip this step.
-Now find the least electronegative atom in the compound and place it as the central atom.
Here C is the least electronegative than O, as the C is far away from F than O, so C is taken as the central atom and the O is distributed as the outside atoms.
-Now fill two electrons in between the two atoms to show the chemical bonding with the atoms.
-Now complete the octet for the outside atoms i.e. give eight electrons for the outside atoms.
From the $16{{e}^{-}}s$, give the two O eight electrons each so that it completes its octet configuration.
-And now check the octet configuration for all the atoms in the compound if the central atom lacks the octet configuration,then shift the electron pair towards the central atom to form multiple bonds.
Here if we see the distribution of electrons as said earlier, the C lacks electrons.And hence transfer the electron pairs from the outside atom towards the central atom,so that all the electrons will achieve the octet configuration.
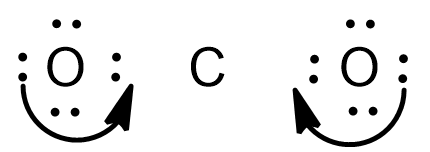
And the final Lewis dot structure of $C{{O}_{2}}$ is as follows:
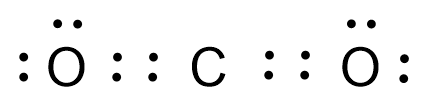
In this structure all the atoms have eight electrons, hence the criteria of octet configuration is satisfied.
Note: The Lewis dot structure gives an idea about the bond angle and about the structure of the compound.Here the structure of carbon dioxide is linear and the O-C-O bond angle is ${{180}^{\circ }}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




