
Draw a neat and labelled diagram of blast furnace.
Answer
573.9k+ views
Hint: The purpose of a blast furnace is generally the chemical reduction and physical conversion of iron oxides into liquid iron termed as "hot metal". The blast furnace is a huge, steel stack lined with refractory brick, where iron ore, coke and limestone are dumped into the top, and preheated air is blown into the bottom.
Complete step by step answer:
The working of the blast furnace is given below.
In the blast furnace raw materials require \[6-8{ }hours\] to descend to the bottom of the furnace where they become the final product of liquid iron and liquid slag. These liquid products are drained from the furnace at regular intervals. The hot air which was being blown from the bottom of the furnace comes up to the top in a span of \[6-8{ }seconds\] after going through several chemical reactions.
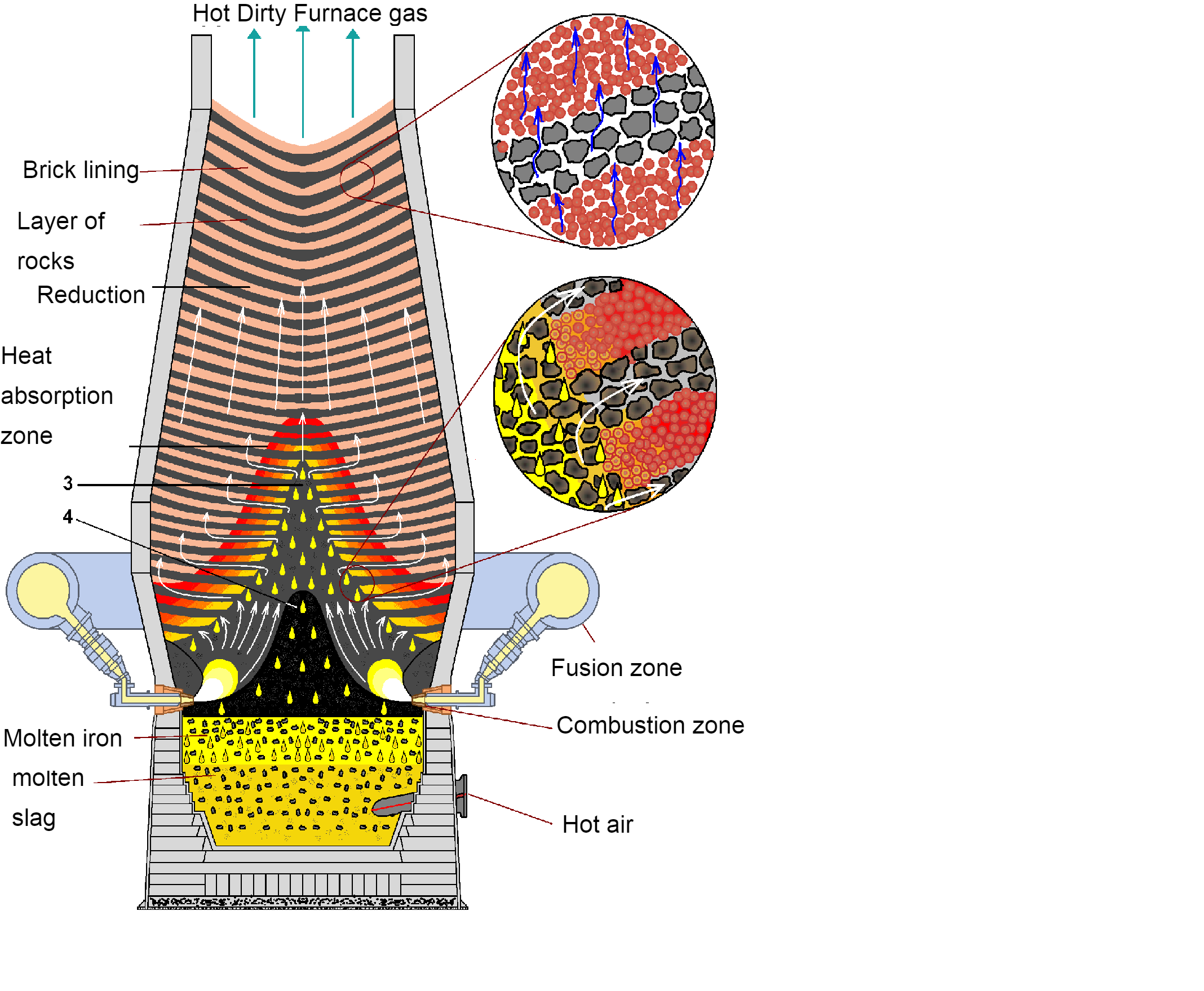
The production of coke is done by using a mixture of coals. At first the coal is ground and crushed into a form of powder and then is transferred into an oven. As the heating of the oven starts the coal is being cooked, therefore most of the volatile matter like the tar and oil are removed. The cooked coal, which is termed as coke, is removed from the oven after a timespan of \[18-24{ }hours\]. The strong pieces of coke which have a high energy value provide gases, heat and permeability which are necessarily needed in order to melt and reduce the iron sinter ore, and pellets.
The final raw material which is being used in the process of ironmaking in limestone. The limestone is removed from those surfaces of earth by blasting it with explosives. It is then screened and crushed to a size which ranges from \[0.5{ }inch-{ }1.5{ }inch\] to become a flux of blast furnace. This flux can be pure with high content of dolomitic limestone, calcium limestone, containing magnesia or a blend of the two types of limestone.
The iron ore, in the form of pellets and sinter are being reduced which simply means that the oxygen in the iron oxides is being removed by a number or a series of chemical reactions. These reactions are represented as follows:
1) \[3F{{e}_{2}}{{O}_{3}}+CO=C{{O}_{2}}+2F{{e}_{3}}{{O}_{4}}\] Begins at \[450{}^\circ C\]
2) \[F{{e}_{3}}{{O}_{4}}+CO=C{{O}_{2}}+3{ }FeO\] Begins at \[600{}^\circ C\]
3) \[FeO+CO{ }=C{{O}_{2}}+Fe\]
Note: Once a blast furnace gets started it will constantly run for four to ten years with only short stops in order to perform some planned maintenance. The coke produced in this process has \[90-93%\] carbon content, some sulphur and ash but if compared to raw coal, it is very strong.
Complete step by step answer:
The working of the blast furnace is given below.
In the blast furnace raw materials require \[6-8{ }hours\] to descend to the bottom of the furnace where they become the final product of liquid iron and liquid slag. These liquid products are drained from the furnace at regular intervals. The hot air which was being blown from the bottom of the furnace comes up to the top in a span of \[6-8{ }seconds\] after going through several chemical reactions.

The production of coke is done by using a mixture of coals. At first the coal is ground and crushed into a form of powder and then is transferred into an oven. As the heating of the oven starts the coal is being cooked, therefore most of the volatile matter like the tar and oil are removed. The cooked coal, which is termed as coke, is removed from the oven after a timespan of \[18-24{ }hours\]. The strong pieces of coke which have a high energy value provide gases, heat and permeability which are necessarily needed in order to melt and reduce the iron sinter ore, and pellets.
The final raw material which is being used in the process of ironmaking in limestone. The limestone is removed from those surfaces of earth by blasting it with explosives. It is then screened and crushed to a size which ranges from \[0.5{ }inch-{ }1.5{ }inch\] to become a flux of blast furnace. This flux can be pure with high content of dolomitic limestone, calcium limestone, containing magnesia or a blend of the two types of limestone.
The iron ore, in the form of pellets and sinter are being reduced which simply means that the oxygen in the iron oxides is being removed by a number or a series of chemical reactions. These reactions are represented as follows:
1) \[3F{{e}_{2}}{{O}_{3}}+CO=C{{O}_{2}}+2F{{e}_{3}}{{O}_{4}}\] Begins at \[450{}^\circ C\]
2) \[F{{e}_{3}}{{O}_{4}}+CO=C{{O}_{2}}+3{ }FeO\] Begins at \[600{}^\circ C\]
3) \[FeO+CO{ }=C{{O}_{2}}+Fe\]
Note: Once a blast furnace gets started it will constantly run for four to ten years with only short stops in order to perform some planned maintenance. The coke produced in this process has \[90-93%\] carbon content, some sulphur and ash but if compared to raw coal, it is very strong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




