
Draw a neat diagram showing action of scheme on metal and label the parts
Answer
487.5k+ views
Hint: When metals react with steam, solid metal oxide and hydrogen gas are formed. If the reaction is faster, that means the metal is more reactive in nature. Its surface generates an aluminium oxide protective layer, which keeps water away from the metal beneath. Metals like iron, aluminium, and zinc do not show reaction with hot or cold water; instead, they react with steam to produce a metal oxide and hydrogen.
Complete Answer:
The diagram shows the Action of steam on metal:
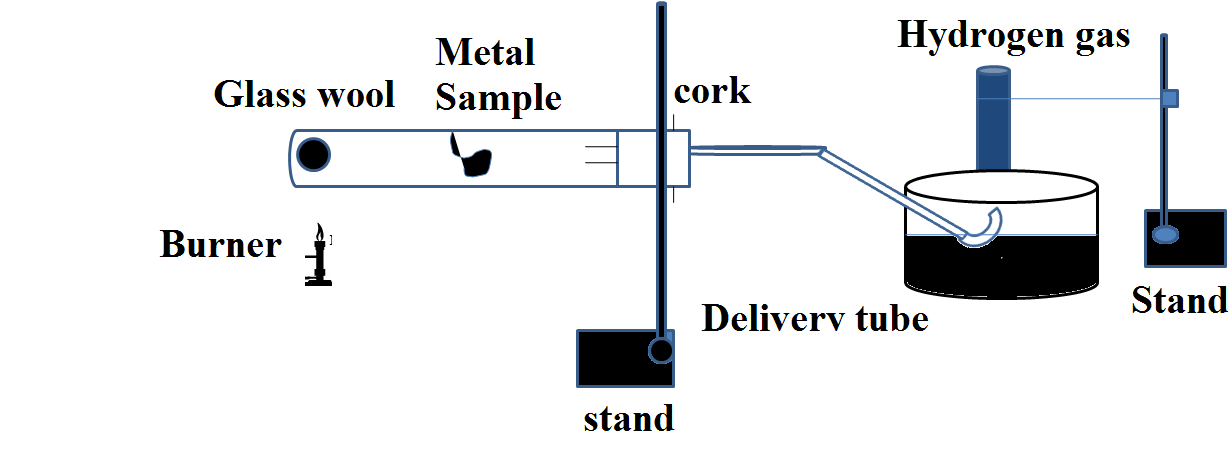
Some metals react with water as well, although they do so in different ways than they do with oxygen. Placing metals in a trough of cold water allows us to examine their response with water. We can also see the differences in metal reactions by utilising steam (hot water) instead of cold water.
Additional Information:
Cold water reacts violently with metals like potassium and sodium. The interaction between sodium and potassium is so strong and exothermic that the released hydrogen immediately catches fire. Because the hydrogen gas bubbles that form adhere to the metal's surface, calcium begins to float. Magnesium does not react when it comes into contact with cold water. Magnesium hydroxide and hydrogen are produced when it reacts with hot water. Due to hydrogen gas bubbles adhering to its surface, it also begins to float.
Note:
When metals come into contact with water, they form metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve and generate metal hydroxide as a result. However, not all metals react with water. Lead, copper, silver, and gold are metals that do not react with water.
Metal + Water $\to$ Metal oxide + Hydrogen
Metal oxide + Water $\to$ Metal hydroxide
Complete Answer:
The diagram shows the Action of steam on metal:

Some metals react with water as well, although they do so in different ways than they do with oxygen. Placing metals in a trough of cold water allows us to examine their response with water. We can also see the differences in metal reactions by utilising steam (hot water) instead of cold water.
Additional Information:
Cold water reacts violently with metals like potassium and sodium. The interaction between sodium and potassium is so strong and exothermic that the released hydrogen immediately catches fire. Because the hydrogen gas bubbles that form adhere to the metal's surface, calcium begins to float. Magnesium does not react when it comes into contact with cold water. Magnesium hydroxide and hydrogen are produced when it reacts with hot water. Due to hydrogen gas bubbles adhering to its surface, it also begins to float.
Note:
When metals come into contact with water, they form metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve and generate metal hydroxide as a result. However, not all metals react with water. Lead, copper, silver, and gold are metals that do not react with water.
Metal + Water $\to$ Metal oxide + Hydrogen
Metal oxide + Water $\to$ Metal hydroxide
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




