
How can I draw a perspective formula for \[\left( \text{R} \right)-1,2-\] dibromobutane?
Answer
548.1k+ views
Hint: We know that molecules are arranged in three dimensional manner in such a way that minimum repulsion occurs between the atoms or groups of atoms which are attached with each other. Several scientists gave representation of three dimensional structure in two dimensional so that we can easily understand the structure of the particular molecule by the help of it.
Complete step by step answer:
There are two different stereoisomers for a molecule that has a centre of chirality; absolute configurations in such case are expressed in terms of $R$ and $S$ configuration. This nomenclature indicates the configuration of the chirality centre.
This device is based on the actual $3-$ dimensional formula of the compound or the perspective formula.
First Step includes: draw the butane chain.
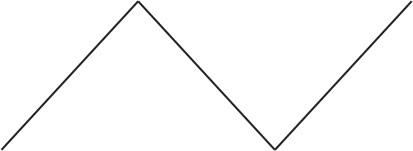
Then from the name we can conclude that the functional group is attached to $\text{C-1}$ and $\text{C-2}$ carbon.
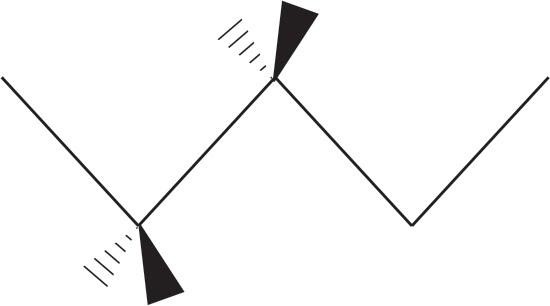
Wedge and dashes are added to $\text{C}-1$ and \[\text{C}-2\] .
Now Add $\text{Br}$ to solid bonds and $\text{H}$ atoms to other bonds of $\text{C}-1$
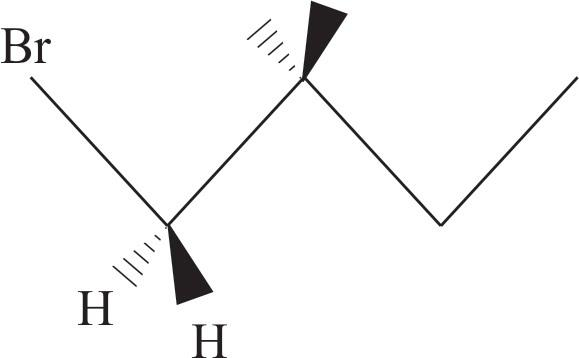
Now add the second $\text{Br}$ to dash on \[\text{C}-2\] and $\text{H}-$ atom to wedge bond.
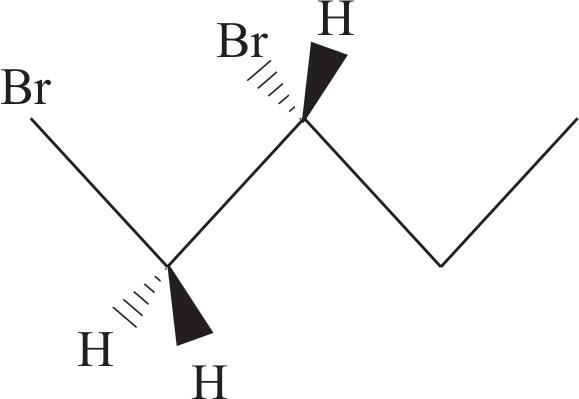
This the perspective formula for $(R)$ $1-2-$ dibromobutane
Note: Perspective formula is the formula in which we tried to make three dimensional structure in two dimensional structure. In the perspective formula of the carbon molecule it was assumed that two of the four valencies of carbon atom are present on the plane. Out of four, one valency is present above the plane and the last valency is present below the plane.
Complete step by step answer:
There are two different stereoisomers for a molecule that has a centre of chirality; absolute configurations in such case are expressed in terms of $R$ and $S$ configuration. This nomenclature indicates the configuration of the chirality centre.
This device is based on the actual $3-$ dimensional formula of the compound or the perspective formula.
First Step includes: draw the butane chain.

Then from the name we can conclude that the functional group is attached to $\text{C-1}$ and $\text{C-2}$ carbon.

Wedge and dashes are added to $\text{C}-1$ and \[\text{C}-2\] .
Now Add $\text{Br}$ to solid bonds and $\text{H}$ atoms to other bonds of $\text{C}-1$

Now add the second $\text{Br}$ to dash on \[\text{C}-2\] and $\text{H}-$ atom to wedge bond.

This the perspective formula for $(R)$ $1-2-$ dibromobutane
Note: Perspective formula is the formula in which we tried to make three dimensional structure in two dimensional structure. In the perspective formula of the carbon molecule it was assumed that two of the four valencies of carbon atom are present on the plane. Out of four, one valency is present above the plane and the last valency is present below the plane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




