
Draw an electron dot diagram to show the formation of ammonium ion[Atomic number: N=7 and H=1].
Answer
524.5k+ views
Hint: Ammonium ion consists of nitrogen and hydrogen atoms. It has one nitrogen atom and 4 hydrogen atoms.
Complete step-by-step answer:
-Ammonia consists of one nitrogen and three hydrogen. Nitrogen has five valence electrons. Out of which 3 electrons of nitrogen form a covalent bond with hydrogen, with sharing of one electron of nitrogen and one electron of hydrogen. There are three such bonds. So, two electrons are left as a lone pair. This is the structure of ammonia or \[N{{H}_{3}}\].
-In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is shown below.
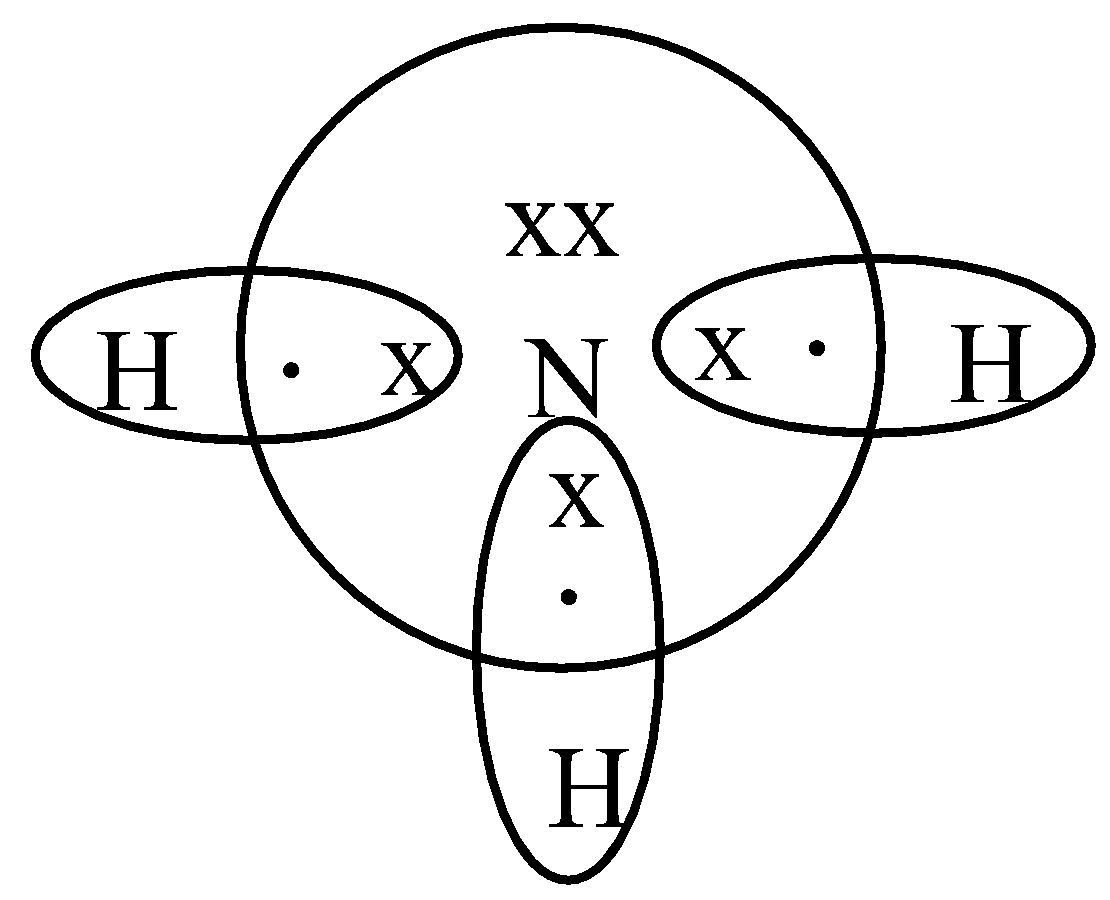
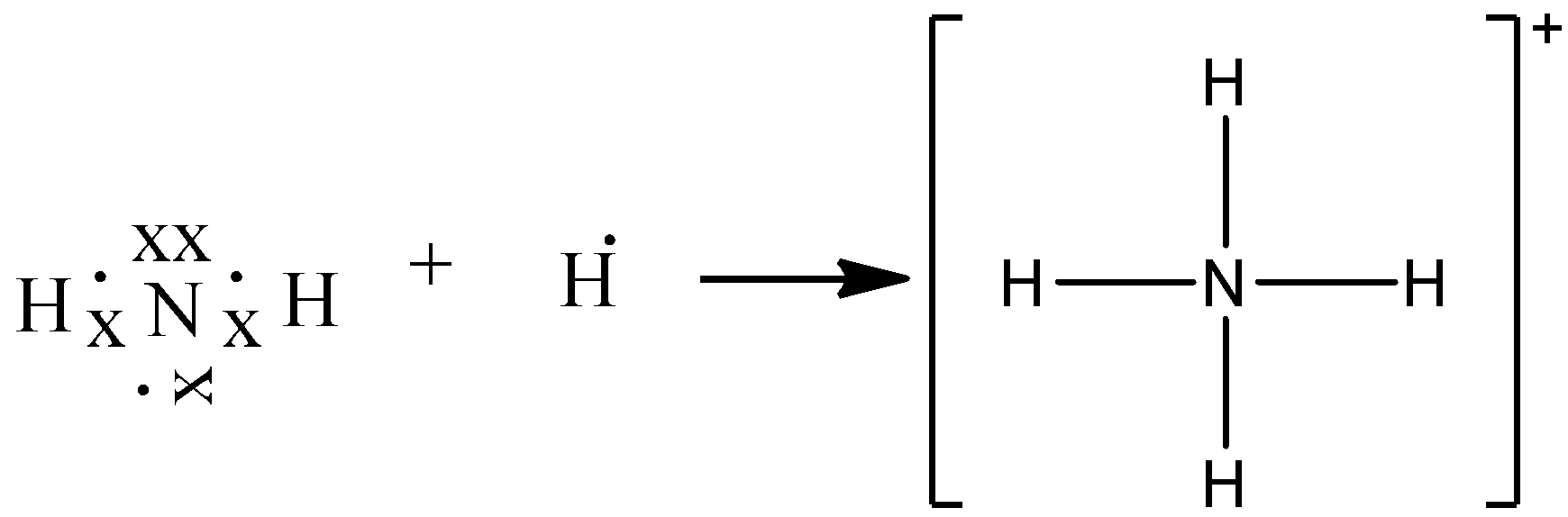
Additional Information:
-Both ammonia and ammonium have tetrahedral structure. When ammonia reacts with water ammonium ion is formed by taking up a proton from water. Ammonium ion is the one which can form nontoxic salts. Under normal conditions, both ammonia and ammonium ions will be present in normal water.
-Electron dot diagrams can show how the electrons are involved in a bond formation. It is a diagram in which valence electrons of an atom are shown as dots and how they are distributed around the element’s symbol. Usually the dot diagrams are the same for each element in their representative element groups.
Note: Ammonia and ammonium ions are different. Ammonium ion is a positive species while ammonia is neutral; it has no charge on it.
Complete step-by-step answer:
-Ammonia consists of one nitrogen and three hydrogen. Nitrogen has five valence electrons. Out of which 3 electrons of nitrogen form a covalent bond with hydrogen, with sharing of one electron of nitrogen and one electron of hydrogen. There are three such bonds. So, two electrons are left as a lone pair. This is the structure of ammonia or \[N{{H}_{3}}\].
-In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is shown below.


Ammonium Ion
Additional Information:
-Both ammonia and ammonium have tetrahedral structure. When ammonia reacts with water ammonium ion is formed by taking up a proton from water. Ammonium ion is the one which can form nontoxic salts. Under normal conditions, both ammonia and ammonium ions will be present in normal water.
-Electron dot diagrams can show how the electrons are involved in a bond formation. It is a diagram in which valence electrons of an atom are shown as dots and how they are distributed around the element’s symbol. Usually the dot diagrams are the same for each element in their representative element groups.
Note: Ammonia and ammonium ions are different. Ammonium ion is a positive species while ammonia is neutral; it has no charge on it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




