
Draw an electron dot structure of the following molecule. (Without showing the circle): Methane.
Answer
520.8k+ views
Hint :The central atom in a methane molecule is carbon. In electron dot structure, valence electrons of the element are represented. So, Carbon has 4 electrons in its electron dot structure and hydrogen has only one electron. Carbon and hydrogen share these electrons to form a single bond.
Complete Step By Step Answer:
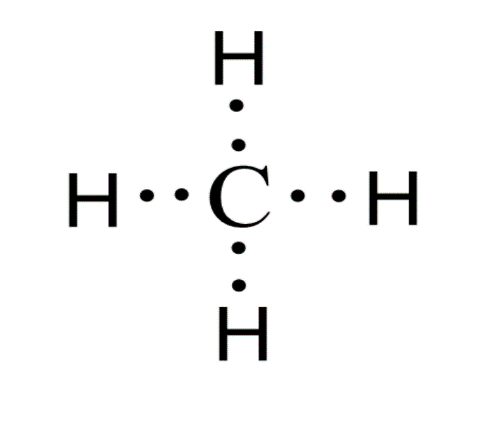
Lewis electron dot diagram represents the valence electrons of an atom using dots around the symbol of the element. The number of dots around the element are equal to the number of valence electrons in the atom. The electrons present in the outermost shell of an atom are called valence electrons. Lewis dot structures are also called Lewis dot formulas. They also show the bonding between the atoms present in a molecule and also the lone pairs of electrons present in the molecule. The electron dot structure of methane is shown below:
Note :
In a methane molecule, covalent bonds are present between four hydrogen atoms and the central carbon atom. Methane is a gas and it is colourless. Single covalent bonds are present in this molecule. Such types of compounds are bad conductors of electricity as they form covalent compounds which do not have free ions or free electrons. The electron dot structure also shows us the unpaired electrons that are present in the molecule. In this structure, each dot represents an electron.
Complete Step By Step Answer:
Lewis electron dot diagram represents the valence electrons of an atom using dots around the symbol of the element. The number of dots around the element are equal to the number of valence electrons in the atom. The electrons present in the outermost shell of an atom are called valence electrons. Lewis dot structures are also called Lewis dot formulas. They also show the bonding between the atoms present in a molecule and also the lone pairs of electrons present in the molecule. The electron dot structure of methane is shown below:

Note :
In a methane molecule, covalent bonds are present between four hydrogen atoms and the central carbon atom. Methane is a gas and it is colourless. Single covalent bonds are present in this molecule. Such types of compounds are bad conductors of electricity as they form covalent compounds which do not have free ions or free electrons. The electron dot structure also shows us the unpaired electrons that are present in the molecule. In this structure, each dot represents an electron.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




