
Draw electron dot representation of $ \text{CC}{{\text{l}}_{\text{4}}} $ .
Answer
552k+ views
Hint: A Lewis electron dot diagram is a representation of the valence shell electrons of an atom that uses dots around the symbol of the central element as a representation of the electrons. The bonds are represented as pairs of electrons shared.
Complete Stepwise solution
In carbon tetrachloride or $ \text{CC}{{\text{l}}_{\text{4}}} $ , the central element is carbon, whose valence shell has four electrons and the number of electrons in the valence shell of the chlorine is seven. So the carbon atom needs four more electrons in the valence shell while chlorine needs only one more electron, hence they share the electrons to form four covalent bonds. Each carbon atom forms one covalent bond with each chlorine atom. The electronic representation of the compound is as follows:
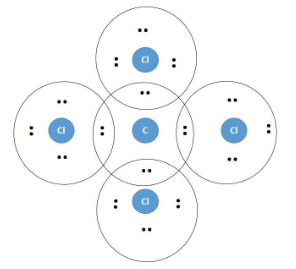
In the above figure, the carbon atom is seen to share four of its valence electrons with one valence electron of the chlorine atom and thus form a strong covalent bond. This also leads to fulfilment of their octet.
Note
The Lewis electron dot configuration is a way to represent the molecular bond formation of a compound in a pictorial manner. But this technique is not used as much as it was used before nowadays. This is because these structures are two-dimensional figures that cannot predict the stereochemistry of a compound or the arrangement of the different atoms in the molecule in space around the atom.
Nonetheless, these structures provide preliminary knowledge about the bonding pattern and hence are useful to predict the bonding nature of a covalent or ionic molecule.
Complete Stepwise solution
In carbon tetrachloride or $ \text{CC}{{\text{l}}_{\text{4}}} $ , the central element is carbon, whose valence shell has four electrons and the number of electrons in the valence shell of the chlorine is seven. So the carbon atom needs four more electrons in the valence shell while chlorine needs only one more electron, hence they share the electrons to form four covalent bonds. Each carbon atom forms one covalent bond with each chlorine atom. The electronic representation of the compound is as follows:

In the above figure, the carbon atom is seen to share four of its valence electrons with one valence electron of the chlorine atom and thus form a strong covalent bond. This also leads to fulfilment of their octet.
Note
The Lewis electron dot configuration is a way to represent the molecular bond formation of a compound in a pictorial manner. But this technique is not used as much as it was used before nowadays. This is because these structures are two-dimensional figures that cannot predict the stereochemistry of a compound or the arrangement of the different atoms in the molecule in space around the atom.
Nonetheless, these structures provide preliminary knowledge about the bonding pattern and hence are useful to predict the bonding nature of a covalent or ionic molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




