
Draw $${H_2}S{O_4}$$ lewis dot structure.
Answer
558.3k+ views
Hint: The Lewis dot structure is defined as the graphic representation of the electrons which are distributed around the atom. It helps us in determining the number of bonds that can be made around the atom. It also predicts the nature or the type of bond around the atom. It further helps in the prediction of geometry of atoms.
Complete step by step answer:
We can also find the formal charge by the help of the Lewis dot structure. So the valence electrons present in the sulphuric acid is 32 electrons or 16 electron pairs as sulphur has 6 valence electrons, the valence electrons in hydrogen is 1 and in oxygen there are 6 valence electrons. So the structure of sulphuric acid is the following
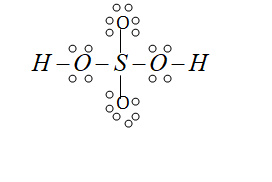
Here we can make the double bond with the oxygen and sulphur molecules because the formal charge on them is present. so the final structure or we can say the more stable structure is the following:
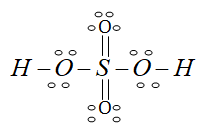
So the above structure is the lewis dot structure for the sulphuric acid.
The lewis structure helps us in learning the oxidation states and valencies.
Note: The formal charge is considered as the theoretical charge because it does not indicate the real charge separation present in the molecule. It also helps in determining the structures which have lowest energy. It helps in the prediction of the major product that is formed in the reaction. The formal charge which is present on the lowest energy structure is the lowest.
Complete step by step answer:
We can also find the formal charge by the help of the Lewis dot structure. So the valence electrons present in the sulphuric acid is 32 electrons or 16 electron pairs as sulphur has 6 valence electrons, the valence electrons in hydrogen is 1 and in oxygen there are 6 valence electrons. So the structure of sulphuric acid is the following
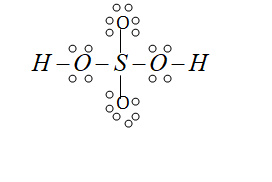
Here we can make the double bond with the oxygen and sulphur molecules because the formal charge on them is present. so the final structure or we can say the more stable structure is the following:

So the above structure is the lewis dot structure for the sulphuric acid.
The lewis structure helps us in learning the oxidation states and valencies.
Note: The formal charge is considered as the theoretical charge because it does not indicate the real charge separation present in the molecule. It also helps in determining the structures which have lowest energy. It helps in the prediction of the major product that is formed in the reaction. The formal charge which is present on the lowest energy structure is the lowest.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




