
Draw Lewis structure of tetracyanoethylene and point the total number of sigma and pi bonds.
Answer
573.9k+ views
Hint: Lewis structure shows a bonding between the atoms of a molecule. It is an electron dot structure that can be drawn for covalently bonded molecules as well as coordination compounds. It shows the position of an atom in different structures.
Complete step by step answer:
As we know, a triple bond contains two pi bonds and one sigma bond.
The structure of tetracyanoethylene molecule is:
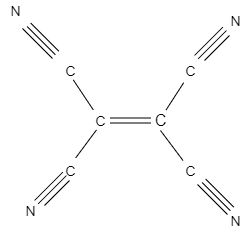
Here, as you can see, there are nine sigma bonds and nine pi bonds. The nine sigma bonds consist of four between the four $C\equiv N$ groups, and four between the $C-C$ bond attached to cyanide and one sigma bond of ethylene, whereas there are nine pi bonds. Two pi bonds lie between each $C\equiv N$ (cyanide) and therefore it will be eight pi bonds and one bond lies between $C=C$ bond.
So the total number of pi bonds is nine.
Additional information:
Tetracyanoethylene is an organic compound which has a formula . It is a member of cyanocarbons. It is the first percy and olefin that is prepared. It is a colorless compound and a versatile compound with exceptional reactivity.
Methods of preparation:
It is prepared by brominating malononitrile in the presence of potassium bromide.
Sigma bond is defined as the strongest bond of covalent chemical bond. It is defined basically for diatomic molecules. It is formed from head to head overlapping between the atomic orbitals. It is a symmetrical bond and considered as the strongest type of bond because of direct overlapping of orbitals and the electrons present in these bonds are known as sigma electrons.Pi bonds are defined as covalent chemical bonds in which two lobes of an orbital on one atom, it overlaps two lobes of another atom. This overlapping occurs laterally. Double and triple bonds contain pi bonds but pi bonds are not present in single bonds.
Note: Cyanide contains triple bonds where there are two pi bonds and one sigma bond. Ethylene carbon – carbon double bond contains one pi bond and one sigma bond.Tetracyanoethylene is a member of cyanocarbons that contain several cyanide functional groups.
Complete step by step answer:
As we know, a triple bond contains two pi bonds and one sigma bond.
The structure of tetracyanoethylene molecule is:

Here, as you can see, there are nine sigma bonds and nine pi bonds. The nine sigma bonds consist of four between the four $C\equiv N$ groups, and four between the $C-C$ bond attached to cyanide and one sigma bond of ethylene, whereas there are nine pi bonds. Two pi bonds lie between each $C\equiv N$ (cyanide) and therefore it will be eight pi bonds and one bond lies between $C=C$ bond.
So the total number of pi bonds is nine.
Additional information:
Tetracyanoethylene is an organic compound which has a formula . It is a member of cyanocarbons. It is the first percy and olefin that is prepared. It is a colorless compound and a versatile compound with exceptional reactivity.
Methods of preparation:
It is prepared by brominating malononitrile in the presence of potassium bromide.
Sigma bond is defined as the strongest bond of covalent chemical bond. It is defined basically for diatomic molecules. It is formed from head to head overlapping between the atomic orbitals. It is a symmetrical bond and considered as the strongest type of bond because of direct overlapping of orbitals and the electrons present in these bonds are known as sigma electrons.Pi bonds are defined as covalent chemical bonds in which two lobes of an orbital on one atom, it overlaps two lobes of another atom. This overlapping occurs laterally. Double and triple bonds contain pi bonds but pi bonds are not present in single bonds.
Note: Cyanide contains triple bonds where there are two pi bonds and one sigma bond. Ethylene carbon – carbon double bond contains one pi bond and one sigma bond.Tetracyanoethylene is a member of cyanocarbons that contain several cyanide functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




