
Draw Lewis structures for \[{{C}_{2}}^{2-}\] ions.
Answer
573.3k+ views
Hint: The Lewis structures, also termed as electron dot diagrams or Lewis-dot diagrams, are pictures that represent the bonding between the atoms of a molecule and a pair of electrons. Each electron is represented by one dot. Two dots which are drawn side by side represent a lone pair of electrons.
Complete step by step answer:
Lewis structures, which is also termed as Lewis-dot diagrams, generally represents the bonding and relationship between the atoms involved in a molecule along with the lone pairs of electrons which are present in the molecule. A compound may have multiple forms of resonating structures which are also all correct Lewis structures.
Sometimes, writing the Lewis structures can become a bit, tricky and somewhat problematic. A compound may have a number of Lewis dot Structures that contribute to the shape of the overall compound, so it is not necessary that one Lewis structure will tell us the overall shape of a molecule. In a Lewis dot structure, an electron is represented as a dot. Whereas a bond, which is made up of two shared electrons, is denoted by two dots among the bonded atoms or a line. Triple bonds and Double bonds are characterised by three and two lines, respectively. Lone pairs present on the outer rims of an atom are denoted by two dots. The electrons represented in a Lewis structure are the electrons which are present in outer-shell, and are termed valence electrons. This is because they are the ones which are involved in chemical reactions.
The total number of electrons which are shown in a Lewis structure is equal to the sum of the numbers of valence electrons present in each individual atom involved in the molecule. Non-valence electrons are not shown in Lewis dot structures. After the total number of available electrons has been determined, electrons must be placed into the structure.
The Lewis structure of \[{{C}_{2}}^{2-}\] is shown below
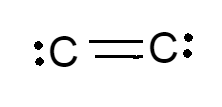
Note: 1. The Lewis structures and Lewis symbols help us visualize the valence electrons of molecules and atoms, whether they exist within bonds or as lone pairs.
2. Lone pairs are unshared, non-bonding electrons pairs present in an atom of a molecule. Although lone pairs are not directly involved in formation of bond, they are always represented in the Lewis dot structures
Complete step by step answer:
Lewis structures, which is also termed as Lewis-dot diagrams, generally represents the bonding and relationship between the atoms involved in a molecule along with the lone pairs of electrons which are present in the molecule. A compound may have multiple forms of resonating structures which are also all correct Lewis structures.
Sometimes, writing the Lewis structures can become a bit, tricky and somewhat problematic. A compound may have a number of Lewis dot Structures that contribute to the shape of the overall compound, so it is not necessary that one Lewis structure will tell us the overall shape of a molecule. In a Lewis dot structure, an electron is represented as a dot. Whereas a bond, which is made up of two shared electrons, is denoted by two dots among the bonded atoms or a line. Triple bonds and Double bonds are characterised by three and two lines, respectively. Lone pairs present on the outer rims of an atom are denoted by two dots. The electrons represented in a Lewis structure are the electrons which are present in outer-shell, and are termed valence electrons. This is because they are the ones which are involved in chemical reactions.
The total number of electrons which are shown in a Lewis structure is equal to the sum of the numbers of valence electrons present in each individual atom involved in the molecule. Non-valence electrons are not shown in Lewis dot structures. After the total number of available electrons has been determined, electrons must be placed into the structure.
The Lewis structure of \[{{C}_{2}}^{2-}\] is shown below
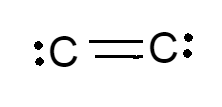
Note: 1. The Lewis structures and Lewis symbols help us visualize the valence electrons of molecules and atoms, whether they exist within bonds or as lone pairs.
2. Lone pairs are unshared, non-bonding electrons pairs present in an atom of a molecule. Although lone pairs are not directly involved in formation of bond, they are always represented in the Lewis dot structures
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




