
Draw structures of the following derivatives.
i) The 2,4-dinitrophenylhydrazone of benzaldehyde
ii) Cyclopropanone oxime
iii) Acetaldehyde Dimethyl Acetal
iv) The semicarbazone of cyclobutanone
v) The ethylene ketal of hexan-3-one
vi) The methyl hemiacetal of formaldehyde
Answer
572.1k+ views
Hint: Use the knowledge of IUPAC nomenclature and common name to draw the structures, focus on the substituents attached and the functional groups of structure. Keep in mind that they are having some special names so should first know about the names and then draw.
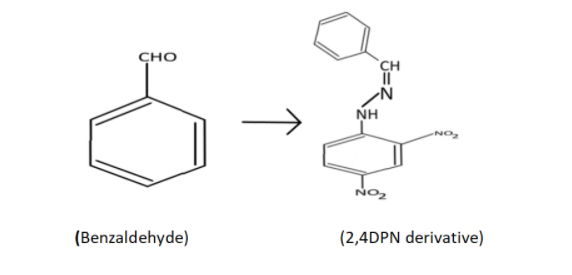
Complete Step by step answer: i) Here, the functional group is aldehyde(-CHO) bonded to benzene, hence benzaldehyde and 2,4-dinitrophenylhydrazone have hydrazone (${{\text{R}}_{\text{1}}}{{\text{R}}_{\text{2}}} - {\text{C}} = {\text{NN}}{{\text{H}}_{\text{2}}}$) and two substituents of nitro (${\text{N}}{{\text{O}}_2}$) are bonded in 2nd and 4th position of the phenyl group. Now it is time for the structure.
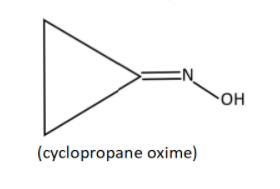
ii) This time an oxime (${{\text{R}}_1}{{\text{R}}_2} - {\text{C}} = {\text{NOH}}$ ) group is bonded with a cyclopropanone. Therefore, (=NOH )group will be bonded with carbon having the ketone functional group. And the structure,
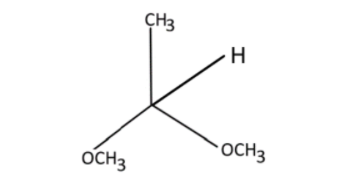
iii) If we breakdown this structure, we can see that this structure has 3 groups. 1-acetaldehyde (${\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO}}$), 2-methyl (-${\text{C}}{{\text{H}}_{\text{3}}}$), and 1-acetal (${{\text{R}}_{\text{2}}}{\text{C}}{\left( {{\text{O}}{{\text{R}}^{\text{'}}}} \right)_{\text{2}}}$). Now let’s draw the structure
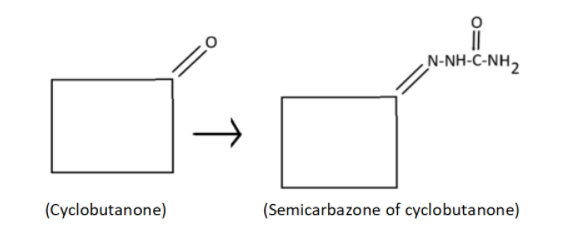
iv) By looking at functional group cyclobutanone we can say it is a ketone, a cyclic ketone with 4 carbon. And semicarbazone. Here’s how the structure
v) Hexane-3-one is a simple ketone compound, and we have to write the ethylene(-CH=CH-) ketal ($ - {{\text{O}}_{\text{2}}}{{\text{R}}_{\text{1}}}{{\text{R}}_{\text{2}}}$) derivate for it. So here what the structure looks like; ${{\text{H}}_{\text{3}}}{\text{C - C}}{{\text{H}}_2}{\text{ - C}}{{\text{H}}_2}{\text{ - }}\mathop {\text{C}}\limits^{\mathop {||}\limits^{\text{O}} } {\text{ - C}}{{\text{H}}_2}{\text{ - C}}{{\text{H}}_{\text{3}}}$ ( Hexan-3-one) $ \to $
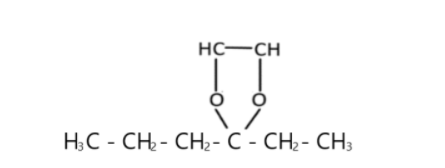
vi) Formaldehyde is the simplest aldehyde (-CHO) there is, it has one carbon and is also called methanal (IUPSC name). Now let’s draw the diagram;
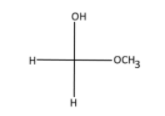
Note: IUPAC names hold importance in chemistry, but more often the common names are used, just like in this question. Therefore, it is also paramount to know the common names of certain compounds. Just read up the common names available in the NCERT book.
Complete Step by step answer: i) Here, the functional group is aldehyde(-CHO) bonded to benzene, hence benzaldehyde and 2,4-dinitrophenylhydrazone have hydrazone (${{\text{R}}_{\text{1}}}{{\text{R}}_{\text{2}}} - {\text{C}} = {\text{NN}}{{\text{H}}_{\text{2}}}$) and two substituents of nitro (${\text{N}}{{\text{O}}_2}$) are bonded in 2nd and 4th position of the phenyl group. Now it is time for the structure.

ii) This time an oxime (${{\text{R}}_1}{{\text{R}}_2} - {\text{C}} = {\text{NOH}}$ ) group is bonded with a cyclopropanone. Therefore, (=NOH )group will be bonded with carbon having the ketone functional group. And the structure,

iii) If we breakdown this structure, we can see that this structure has 3 groups. 1-acetaldehyde (${\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO}}$), 2-methyl (-${\text{C}}{{\text{H}}_{\text{3}}}$), and 1-acetal (${{\text{R}}_{\text{2}}}{\text{C}}{\left( {{\text{O}}{{\text{R}}^{\text{'}}}} \right)_{\text{2}}}$). Now let’s draw the structure

iv) By looking at functional group cyclobutanone we can say it is a ketone, a cyclic ketone with 4 carbon. And semicarbazone. Here’s how the structure

v) Hexane-3-one is a simple ketone compound, and we have to write the ethylene(-CH=CH-) ketal ($ - {{\text{O}}_{\text{2}}}{{\text{R}}_{\text{1}}}{{\text{R}}_{\text{2}}}$) derivate for it. So here what the structure looks like; ${{\text{H}}_{\text{3}}}{\text{C - C}}{{\text{H}}_2}{\text{ - C}}{{\text{H}}_2}{\text{ - }}\mathop {\text{C}}\limits^{\mathop {||}\limits^{\text{O}} } {\text{ - C}}{{\text{H}}_2}{\text{ - C}}{{\text{H}}_{\text{3}}}$ ( Hexan-3-one) $ \to $

vi) Formaldehyde is the simplest aldehyde (-CHO) there is, it has one carbon and is also called methanal (IUPSC name). Now let’s draw the diagram;

Note: IUPAC names hold importance in chemistry, but more often the common names are used, just like in this question. Therefore, it is also paramount to know the common names of certain compounds. Just read up the common names available in the NCERT book.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




