
Draw the atomic structure of chlorine atom and chloride ion.
Answer
558.6k+ views
Hint:The atomic structure consists of electrons, protons and neutrons. The proton and neutron are placed in the nucleus and electrons are placed in different orbits around the nucleus. An ion is an electron rich or electron deficient species of the corresponding atom.
Complete step by step answer:
Chlorine is an atom in the periodic table with atomic number \[17\] and mass number \[35\]. The electronic configuration of chlorine is:
$Cl:1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
The valence shell of chlorine atom is \[3\]. It has a total of \[17\] electrons and \[17\] protons. The number of neutrons =\[35 - 17 = 18\]. The \[17\] protons and \[18\] neutrons are placed in the nucleus of chlorine. The \[17\] electrons are placed in different shells of chlorine atoms.
The shells around the nucleus of an atom are labelled as\[K\] , \[L\] , \[M\] , \[N\] and so on. The first shell \[K = 1\] has two electrons, the second shell \[K = 2\] has eight electrons and the third shell \[M = 3\] has seven electrons.
Thus the atomic structure of chlorine atoms is shown in figure \[1\] .
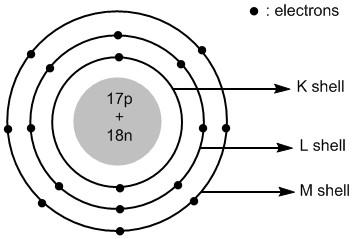
Figure \[1\] : Atomic structure of chlorine atom.
Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is \[18\] . The electronic configuration of chloride ion is:
$C{l^ - }:1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}$
In chloride ion the extra electron is added to the valence shell i.e. third shell or \[M\] shell. Thus the atomic structure of chloride ions is shown in figure \[2\] .
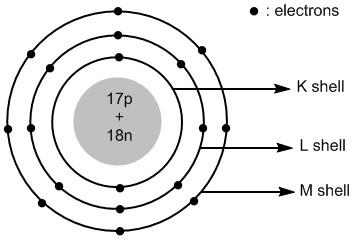
Figure \[2\] : Atomic structure of chloride ions.
Note:
Like the variance of the number of electrons in chlorine and chloride ions, the number of neutrons also varies. Chlorine exists in two isotopes with mass numbers \[35\] and\[37\] . Hence the number of neutrons in \[Cl - 35\] is \[18\] while in \[Cl - 37\] is \[20\].
Complete step by step answer:
Chlorine is an atom in the periodic table with atomic number \[17\] and mass number \[35\]. The electronic configuration of chlorine is:
$Cl:1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
The valence shell of chlorine atom is \[3\]. It has a total of \[17\] electrons and \[17\] protons. The number of neutrons =\[35 - 17 = 18\]. The \[17\] protons and \[18\] neutrons are placed in the nucleus of chlorine. The \[17\] electrons are placed in different shells of chlorine atoms.
The shells around the nucleus of an atom are labelled as\[K\] , \[L\] , \[M\] , \[N\] and so on. The first shell \[K = 1\] has two electrons, the second shell \[K = 2\] has eight electrons and the third shell \[M = 3\] has seven electrons.
Thus the atomic structure of chlorine atoms is shown in figure \[1\] .

Figure \[1\] : Atomic structure of chlorine atom.
Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is \[18\] . The electronic configuration of chloride ion is:
$C{l^ - }:1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}$
In chloride ion the extra electron is added to the valence shell i.e. third shell or \[M\] shell. Thus the atomic structure of chloride ions is shown in figure \[2\] .

Figure \[2\] : Atomic structure of chloride ions.
Note:
Like the variance of the number of electrons in chlorine and chloride ions, the number of neutrons also varies. Chlorine exists in two isotopes with mass numbers \[35\] and\[37\] . Hence the number of neutrons in \[Cl - 35\] is \[18\] while in \[Cl - 37\] is \[20\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




