
Draw the atomic structure of Neon atom and Helium atom.
Answer
569.4k+ views
Hint: Neon and Helium are Noble gases or inert gases which are also sometimes referred to as aerogenes. These are odourless and colourless gases. The atomic number of Helium (He) atoms is 2 and the atomic number of Neon (Ne) is 10.
Compete answer:
Neon atoms and Helium are Noble gases. Neon has 10 electrons in total whereas helium has only two electrons. Both atoms have full outer shells.
The atomic structure of Neon atom is shown below -
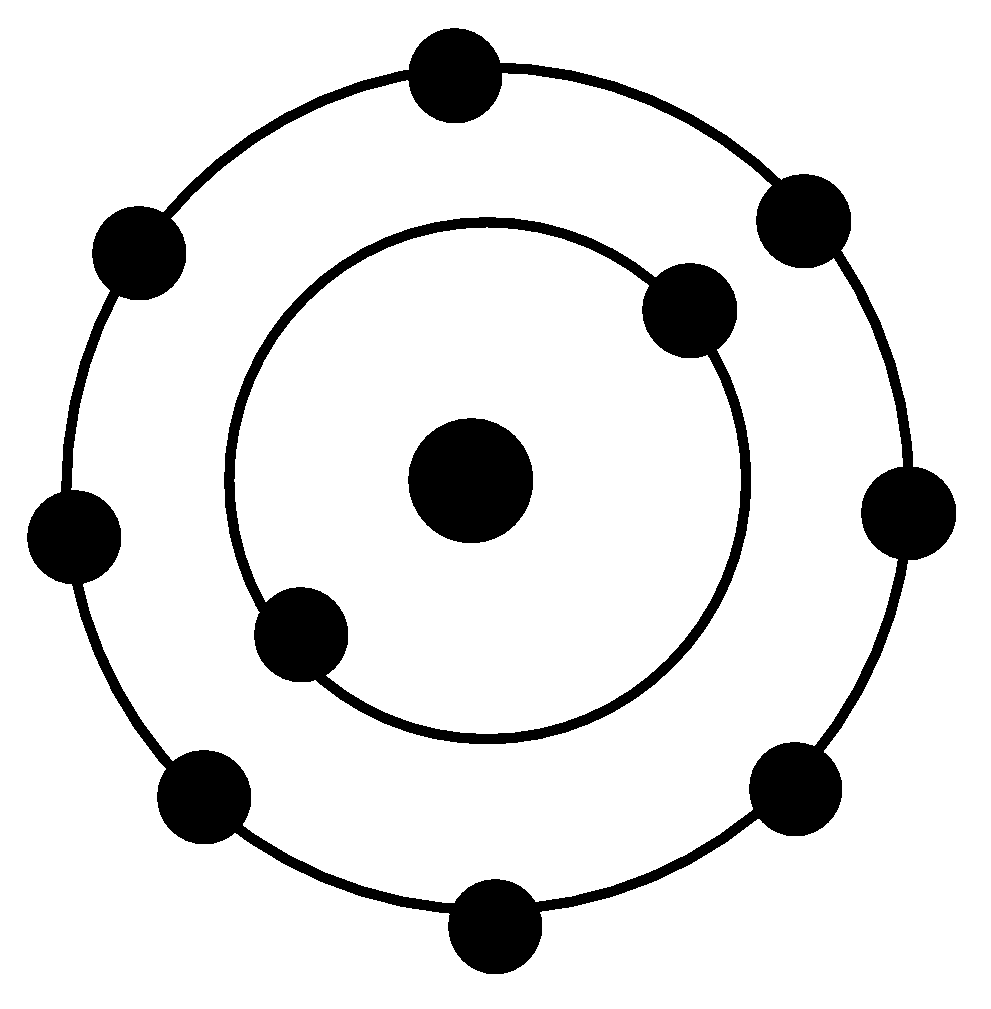
The atomic structure of Helium atom is shown below -
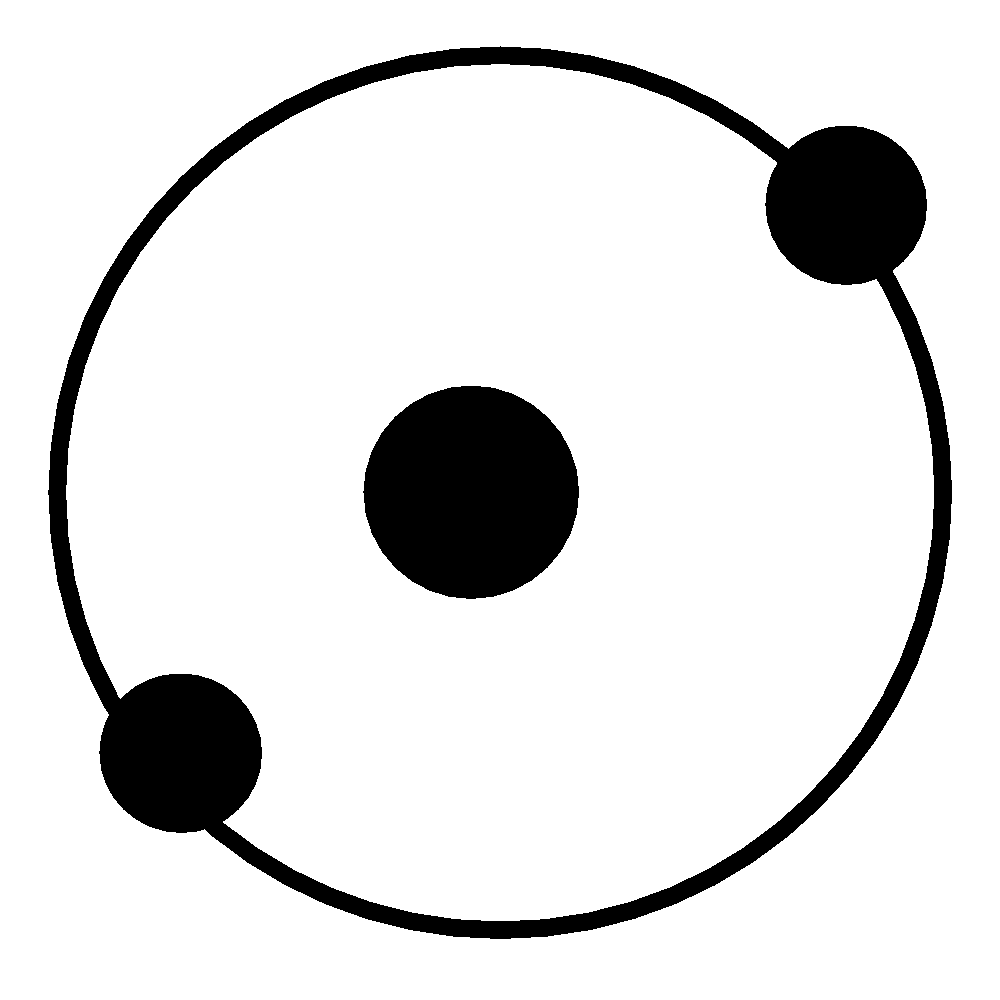
Note: The element Helium was discovered by Pierre Janssen in the year 1895 in Sweden and United Kingdom and this atom has derived its name from the Greek work ‘Helios’ which means ‘Sun’. Whereas the element Neon was discovered by Morris Travers in the year 1898 in United Kingdom. Neon has derived its name from the Greek word ‘Neos’ which means ‘New’. The boiling point of helium is the least when compared to any other liquid. It is used to obtain the lowest temperatures required in lasers. Helium is used in nuclear reactors as a cooling gas and used as a flow-gas in liquid-gas chromatography. It finds its application in airships and helium balloons. Helium balloons are used to check the weather of a particular region. Helium is preferred over hydrogen though hydrogen is cheaper, as helium is readily available and hydrogen is highly inflammable. Hence, due to safety issues helium is preferred in aircraft. Neon is used in discharge tubes which is the reason behind the reddish - orange glow produced by neon lights.
Compete answer:
Neon atoms and Helium are Noble gases. Neon has 10 electrons in total whereas helium has only two electrons. Both atoms have full outer shells.
The atomic structure of Neon atom is shown below -

The atomic structure of Helium atom is shown below -

Note: The element Helium was discovered by Pierre Janssen in the year 1895 in Sweden and United Kingdom and this atom has derived its name from the Greek work ‘Helios’ which means ‘Sun’. Whereas the element Neon was discovered by Morris Travers in the year 1898 in United Kingdom. Neon has derived its name from the Greek word ‘Neos’ which means ‘New’. The boiling point of helium is the least when compared to any other liquid. It is used to obtain the lowest temperatures required in lasers. Helium is used in nuclear reactors as a cooling gas and used as a flow-gas in liquid-gas chromatography. It finds its application in airships and helium balloons. Helium balloons are used to check the weather of a particular region. Helium is preferred over hydrogen though hydrogen is cheaper, as helium is readily available and hydrogen is highly inflammable. Hence, due to safety issues helium is preferred in aircraft. Neon is used in discharge tubes which is the reason behind the reddish - orange glow produced by neon lights.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




