
Draw the diagram of formation of \[HCl\] molecule with orbital overlapping,
Answer
533.4k+ views
Hint: As we know that when orbitals are present in the same phase, they overlap with each other resulting in bond formation which is called positive overlap, when two orbitals of different phases overlap they result in negative overlap and when orbitals do not overlap with each other they result in zero overlap.
Complete step by step answer:
As we know that p-orbital possess a dumbbell shaped orbitals where one of the lobe is positively spin and the other lobe is negative spin and we also know that when two orbitals containing same spin will overlap with each other, they will result in a positive overlap and when the two orbitals containing two different spins overlap, they will result in a negative overlap.
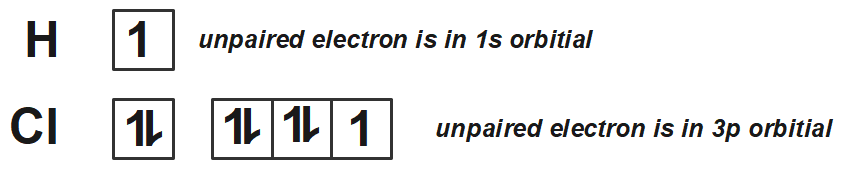
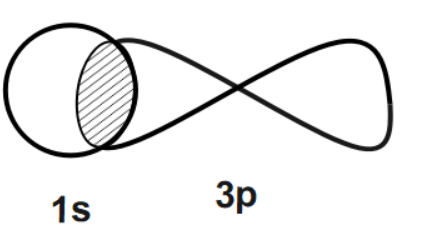
Now, let us talk about each given option one by one. We can see that in the first option, there are two different orbitals that are one p-orbital and one s-orbital possessing positive and negative spins. Thus, it will have a zero overlap where no bonding between these two will not take place.
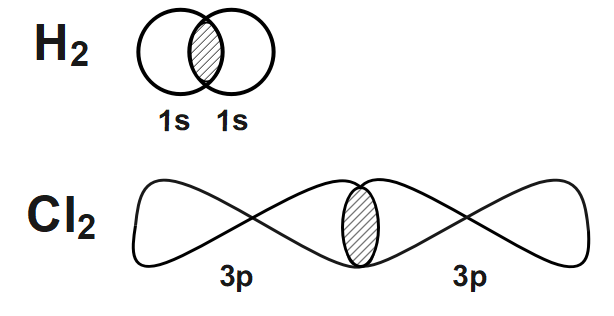
Similarly, in the second and fourth option we can see the negative overlap where positive spin and negative spin containing orbitals are overlapping with each other.So HCl look like.
Note: Always remember that positive overlap will be the result of two similar spins and negative overlap is always shown by two opposite spins. Also, the orbital which is formed by the positive overlap is called a bonding molecular orbital and the orbital which is formed by negative overlap is an antibonding molecular orbital.
Complete step by step answer:
As we know that p-orbital possess a dumbbell shaped orbitals where one of the lobe is positively spin and the other lobe is negative spin and we also know that when two orbitals containing same spin will overlap with each other, they will result in a positive overlap and when the two orbitals containing two different spins overlap, they will result in a negative overlap.

Now, let us talk about each given option one by one. We can see that in the first option, there are two different orbitals that are one p-orbital and one s-orbital possessing positive and negative spins. Thus, it will have a zero overlap where no bonding between these two will not take place.

Similarly, in the second and fourth option we can see the negative overlap where positive spin and negative spin containing orbitals are overlapping with each other.So HCl look like.

Note: Always remember that positive overlap will be the result of two similar spins and negative overlap is always shown by two opposite spins. Also, the orbital which is formed by the positive overlap is called a bonding molecular orbital and the orbital which is formed by negative overlap is an antibonding molecular orbital.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




