
Draw the electron diagram of the formation of chlorine molecules by combining two chlorine atoms?
Answer
567k+ views
Hint: As we know that Electron dot structure is represented as a chemical symbol of a molecule, that is surrounded by a number of dots that represent the valence electron of the atom. Electron dot structure is known as Lewis dot structure, which is used to indicate how the constituent atoms in a molecule are bonded.
Complete answer:
- There are some rules that are needed to be followed while drawing the Lewis dot structure. Let’s discuss in brief about them:
- Write the symbol for the atoms involved, then add the number of all the valence electrons. In case of the negatively charged ions, add the number of charges I the number of valence electrons.
- In the case of positively charged ions, simply subtract the number of charges for the number of valence electrons.
- In the next step, complete the octet of atoms that are attached to the central atom by adding electron pairs. Then add the remaining electrons in pairs on the central atom.
- If the central atom doesn’t have an octet, then form a double bond, or if necessary then form a triple bond.
- We can see the electron diagram of the formation of chlorine molecule by combining two chlorine atoms:
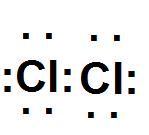
Note: - We should note that there are several limitations of Lewis dot structure: The geometry of the molecules that have a covalent bond can’t be explained by using the structure, it fails to explain the cause of covalent bond formation.
Complete answer:
- There are some rules that are needed to be followed while drawing the Lewis dot structure. Let’s discuss in brief about them:
- Write the symbol for the atoms involved, then add the number of all the valence electrons. In case of the negatively charged ions, add the number of charges I the number of valence electrons.
- In the case of positively charged ions, simply subtract the number of charges for the number of valence electrons.
- In the next step, complete the octet of atoms that are attached to the central atom by adding electron pairs. Then add the remaining electrons in pairs on the central atom.
- If the central atom doesn’t have an octet, then form a double bond, or if necessary then form a triple bond.
- We can see the electron diagram of the formation of chlorine molecule by combining two chlorine atoms:
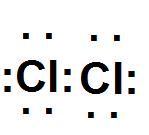
Note: - We should note that there are several limitations of Lewis dot structure: The geometry of the molecules that have a covalent bond can’t be explained by using the structure, it fails to explain the cause of covalent bond formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




