
Draw the electron dot structure of ethane molecule (${{C}_{2}}{{H}_{6}}$ ).
Answer
523.3k+ views
Hint: For the required structure, we need to consider the Lewis structure. For that consider the number of valence electrons for the given molecule and draw the structure. Also, one needs to know that an ethane molecule has 2 carbon atoms and 6 hydrogen atoms. The homologous series formula for alkanes is followed here as Ethane is an alkane. The formula is ${{C}_{n}}{{H}_{2n+2}}$
Complete Step by Step Solution:
The chemical structure for the ethane molecule is given below:
$C{{H}_{3}}-C{{H}_{3}}$
All the bonds present in the ethane molecule are covalent bonds. The Electron dot structure for ethane molecule is:
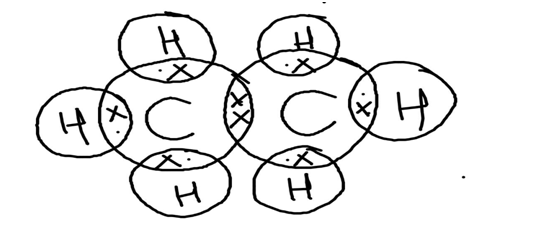
● We know that on the basis of outermost shell electron configuration of Carbon, there is 4 valence electrons for Carbon whereas there is only 1 valence electron for Hydrogen. They are shared as shown in the structure through a covalent bond.
● Each carbon atom is surrounded by three hydrogen atoms as shown.
Note: Some interesting facts about Ethane are that it is the second in the list of Homologous series of Alkane. First one being, Methane whose chemical formula is $C{{H}_{4}}$ , the other ones in this series are Propane (${{C}_{3}}{{H}_{8}}$ ), Butane (${{C}_{4}}{{H}_{10}}$ ) and so on.
Complete Step by Step Solution:
The chemical structure for the ethane molecule is given below:
$C{{H}_{3}}-C{{H}_{3}}$
All the bonds present in the ethane molecule are covalent bonds. The Electron dot structure for ethane molecule is:

● We know that on the basis of outermost shell electron configuration of Carbon, there is 4 valence electrons for Carbon whereas there is only 1 valence electron for Hydrogen. They are shared as shown in the structure through a covalent bond.
● Each carbon atom is surrounded by three hydrogen atoms as shown.
Note: Some interesting facts about Ethane are that it is the second in the list of Homologous series of Alkane. First one being, Methane whose chemical formula is $C{{H}_{4}}$ , the other ones in this series are Propane (${{C}_{3}}{{H}_{8}}$ ), Butane (${{C}_{4}}{{H}_{10}}$ ) and so on.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




