
Draw the electron dot structure of ethyne and also write its structural formula.
Answer
524.9k+ views
Hint: Ethyne is an unsaturated hydrocarbon. It has one triple bond. Once you draw its structural formula, you can replace the bonds with electron pairs to make it an electron dot structure.
Complete step by step answer:
Let us first look into ethyne as a compound before trying to draw its electron dot structure and its structural formula.
> Ethyne is an unsaturated hydrocarbon. From its name we can know that it has two carbon atoms because of “eth” which means two carbons. It is an alkyne which we come to know from “yne”. The general structural formula of an alkyne is ${{C}_{n}}{{H}_{2n-2}}$. As we know the number of carbon atoms to be two, we can derive the structural formula of ethyne with ease. It is as follows:
\[{{C}_{2}}{{H}_{2}}\]
Its common name is acetylene and is a by-product of the extraction of petroleum and natural gas. The hybridisation of the carbons in this compound is $sp$. From this we can predict its geometry and electronegativity. It has a linear geometry and a fifty percent “s” character, making it the most electronegative carbon atom among its other counter parts in alkane and alkene series.
> Acetylene is a colourless gas, since simpler hydrocarbons are mostly gaseous, and is widely used as a fuel and a building block to help produce other chemical compounds.
Now, let us look at the structure of ethyne. As everything is already described we can directly jump onto structure which is as follows:
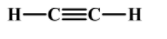
Replacing each of the bonds with a pair of electrons we get the following electron dot structure:
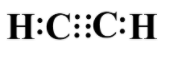
Note: The electron dot structure of any molecule is the same as its Lewis formula. These two words are used interchangeably, so do not get confused with them. Always bear in mind that each bond between two atoms is equal to two electrons. Do not forget to mention the lone pairs if present.
Complete step by step answer:
Let us first look into ethyne as a compound before trying to draw its electron dot structure and its structural formula.
> Ethyne is an unsaturated hydrocarbon. From its name we can know that it has two carbon atoms because of “eth” which means two carbons. It is an alkyne which we come to know from “yne”. The general structural formula of an alkyne is ${{C}_{n}}{{H}_{2n-2}}$. As we know the number of carbon atoms to be two, we can derive the structural formula of ethyne with ease. It is as follows:
\[{{C}_{2}}{{H}_{2}}\]
Its common name is acetylene and is a by-product of the extraction of petroleum and natural gas. The hybridisation of the carbons in this compound is $sp$. From this we can predict its geometry and electronegativity. It has a linear geometry and a fifty percent “s” character, making it the most electronegative carbon atom among its other counter parts in alkane and alkene series.
> Acetylene is a colourless gas, since simpler hydrocarbons are mostly gaseous, and is widely used as a fuel and a building block to help produce other chemical compounds.
Now, let us look at the structure of ethyne. As everything is already described we can directly jump onto structure which is as follows:
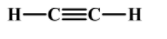
Replacing each of the bonds with a pair of electrons we get the following electron dot structure:
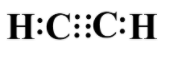
Note: The electron dot structure of any molecule is the same as its Lewis formula. These two words are used interchangeably, so do not get confused with them. Always bear in mind that each bond between two atoms is equal to two electrons. Do not forget to mention the lone pairs if present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




