
Draw the electron dot structures of
(a) ethanoic acid.
(b) \[{H_2}S\].
(c) propanone
(d) \[{F_2}\].
Answer
573.3k+ views
Hint: Lewis structure was given by an American chemist Gilbert Newton Lewis. It is used to represent the structure of atoms and molecules indicating the pair of bonded and non bonded electron pairs.
Complete step by step answer:
Lewis structure or electron dot structure is a representation or a diagram of an atom, molecule or a compound. In these structures the bonds between the atoms and molecules are indicated with straight lines. The electrons on each atom if non- bonded are indicated as lone pairs.
Let us understand the steps for drawing the Lewis structures of the given molecules.
a. The total number of valence electrons has to be added around each atom in a molecule.
b. For anions extra electrons are added to the total number of electrons.
c. For cations extra electrons are subtracted from the total number of electrons.
d. The less electronegative atom is positioned at the centre and surrounded by other atoms.
e. The shared electrons or bonded electrons are shown as single bonds
f. Assign the number of lone pairs first to more electronegative than to less electronegative atoms following octet rule.
g. A double or triple bond is drawn between bonded atoms if required to satisfy the octet of each atom.
Let us draw the Lewis structure for the given molecules using the protocol.
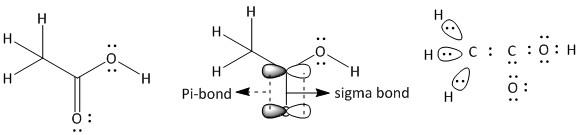
(a) Ethanoic acid. It is a neutral molecule with molecular formula \[C{H_3}COOH\].
The total number of electrons = \[2\] x valence electrons of \[C\] atom + \[4\] x valence electrons of \[H\] atom + \[2\] x valence electrons of \[O\] atom.
= $2 \times 4 + 4 \times 1 + 2 \times 6 = 24$
The given compound is an acid containing a \[\;COOH\] group attached to \[C{H_3}\] group. In \[C{H_3}\], the carbon has four bonded electron pairs, three with hydrogen atoms and one with the carboxyl carbon atom. The three hydrogen atoms have one sigma bond with the methyl carbon atom. The carboxyl carbon has four bonded electrons pairs in which there are three sigma and one pi bond. The pi-bond is between the carbon and one oxygen atom. One of the two oxygen atoms which is bonded by only one sigma bond to carboxyl carbon, has four electron pairs of which two are bonded (one with carbon and one with hydrogen) and two are non-bonded electron pairs. The other oxygen atom bonded by one sigma and one pi-bond has two more non-bonded electron pairs. Thus the structure of ethanoic acid is:
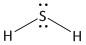
(b) \[{H_2}S\]. It is a neutral molecule.
The total number of electrons = valence electrons of \[S\] atom + \[2\] x valence electrons of \[H\] atom.
= $6 + 2 \times 1 = 8$
Sulphur is the less electronegative atom and is the central atom. It has a total of four electron pairs of which two are bonded and two are non-bonded lone pairs. Hydrogen has a total of one electron pair which is bonded. The two hydrogen atoms form two single bonds. Thus the structure of \[{H_2}S\] is:
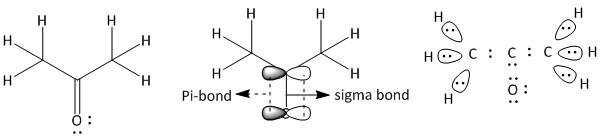
(c) Propanone. It is a neutral molecule with molecular formula \[C{H_3}COC{H_3}\].
The total number of electrons = \[3\] x valence electrons of \[C\] atom + \[4\] x valence electrons of \[H\] atom + valence electrons of \[O\] atom.
= $3 \times 4 + 4 \times 1 + 6 = 22$
The given compound is a ketone containing a \[CO\] group attached to two \[C{H_3}\] groups. In \[C{H_3}\], the carbon has four bonded electron pairs, three are with hydrogen atoms and one with the carbonyl carbon atom. The three hydrogen atoms have one sigma bond with the methyl carbon atom. The carbonyl carbon has four bonded electrons pairs in which there are three sigma and one pi bond. The pi-bond is between the carbon atom and oxygen atom. The oxygen atom has four electron pairs of which two are bonded electron pairs and two are non-bonded electron pairs. Thus the structure of propanone is:
(d) \[{F_2}\]. It is a neutral molecule.
The total number of electrons = \[2\] x valence electrons of \[F\] atom
= $2 \times 7 = 14$
It is a diatomic molecule of fluorine. Each fluorine atom has four electron pairs of which three are non-bonded electron pairs and one is bonded electron pair. The two fluorine atoms are bonded by one sigma bond. Thus the structure of \[{F_2}\] is:
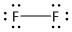
Note: The Lewis structure is also applicable in case of resonance structures. The resonance structures are the electronic representation of molecules showing the movement of non bonded or pi-bonded electrons. The phenomenon is known as resonance. All the resonance structures contribute equally to the overall energy of the molecule.
Complete step by step answer:
Lewis structure or electron dot structure is a representation or a diagram of an atom, molecule or a compound. In these structures the bonds between the atoms and molecules are indicated with straight lines. The electrons on each atom if non- bonded are indicated as lone pairs.
Let us understand the steps for drawing the Lewis structures of the given molecules.
a. The total number of valence electrons has to be added around each atom in a molecule.
b. For anions extra electrons are added to the total number of electrons.
c. For cations extra electrons are subtracted from the total number of electrons.
d. The less electronegative atom is positioned at the centre and surrounded by other atoms.
e. The shared electrons or bonded electrons are shown as single bonds
f. Assign the number of lone pairs first to more electronegative than to less electronegative atoms following octet rule.
g. A double or triple bond is drawn between bonded atoms if required to satisfy the octet of each atom.
Let us draw the Lewis structure for the given molecules using the protocol.
(a) Ethanoic acid. It is a neutral molecule with molecular formula \[C{H_3}COOH\].
The total number of electrons = \[2\] x valence electrons of \[C\] atom + \[4\] x valence electrons of \[H\] atom + \[2\] x valence electrons of \[O\] atom.
= $2 \times 4 + 4 \times 1 + 2 \times 6 = 24$
The given compound is an acid containing a \[\;COOH\] group attached to \[C{H_3}\] group. In \[C{H_3}\], the carbon has four bonded electron pairs, three with hydrogen atoms and one with the carboxyl carbon atom. The three hydrogen atoms have one sigma bond with the methyl carbon atom. The carboxyl carbon has four bonded electrons pairs in which there are three sigma and one pi bond. The pi-bond is between the carbon and one oxygen atom. One of the two oxygen atoms which is bonded by only one sigma bond to carboxyl carbon, has four electron pairs of which two are bonded (one with carbon and one with hydrogen) and two are non-bonded electron pairs. The other oxygen atom bonded by one sigma and one pi-bond has two more non-bonded electron pairs. Thus the structure of ethanoic acid is:

(b) \[{H_2}S\]. It is a neutral molecule.
The total number of electrons = valence electrons of \[S\] atom + \[2\] x valence electrons of \[H\] atom.
= $6 + 2 \times 1 = 8$
Sulphur is the less electronegative atom and is the central atom. It has a total of four electron pairs of which two are bonded and two are non-bonded lone pairs. Hydrogen has a total of one electron pair which is bonded. The two hydrogen atoms form two single bonds. Thus the structure of \[{H_2}S\] is:

(c) Propanone. It is a neutral molecule with molecular formula \[C{H_3}COC{H_3}\].
The total number of electrons = \[3\] x valence electrons of \[C\] atom + \[4\] x valence electrons of \[H\] atom + valence electrons of \[O\] atom.
= $3 \times 4 + 4 \times 1 + 6 = 22$
The given compound is a ketone containing a \[CO\] group attached to two \[C{H_3}\] groups. In \[C{H_3}\], the carbon has four bonded electron pairs, three are with hydrogen atoms and one with the carbonyl carbon atom. The three hydrogen atoms have one sigma bond with the methyl carbon atom. The carbonyl carbon has four bonded electrons pairs in which there are three sigma and one pi bond. The pi-bond is between the carbon atom and oxygen atom. The oxygen atom has four electron pairs of which two are bonded electron pairs and two are non-bonded electron pairs. Thus the structure of propanone is:

(d) \[{F_2}\]. It is a neutral molecule.
The total number of electrons = \[2\] x valence electrons of \[F\] atom
= $2 \times 7 = 14$
It is a diatomic molecule of fluorine. Each fluorine atom has four electron pairs of which three are non-bonded electron pairs and one is bonded electron pair. The two fluorine atoms are bonded by one sigma bond. Thus the structure of \[{F_2}\] is:
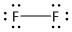
Note: The Lewis structure is also applicable in case of resonance structures. The resonance structures are the electronic representation of molecules showing the movement of non bonded or pi-bonded electrons. The phenomenon is known as resonance. All the resonance structures contribute equally to the overall energy of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




