
How can I draw the following ethers: 1-propoxypentane, 2-ethoxybutane, 1-methoxy-4-chlorohexane and 3-butoxy-2,4-dimethyloctane?
Answer
537.3k+ views
Hint: First of all, draw the main chain of the given compound. Then attach the alkoxy group to the particular carbon number mentioned in the name.
Complete step by step solution: Ethers can be categorized as organic compounds where 1 oxygen atom is connected by 2 alkyl or aryl groups. They have the general formula as follows:-
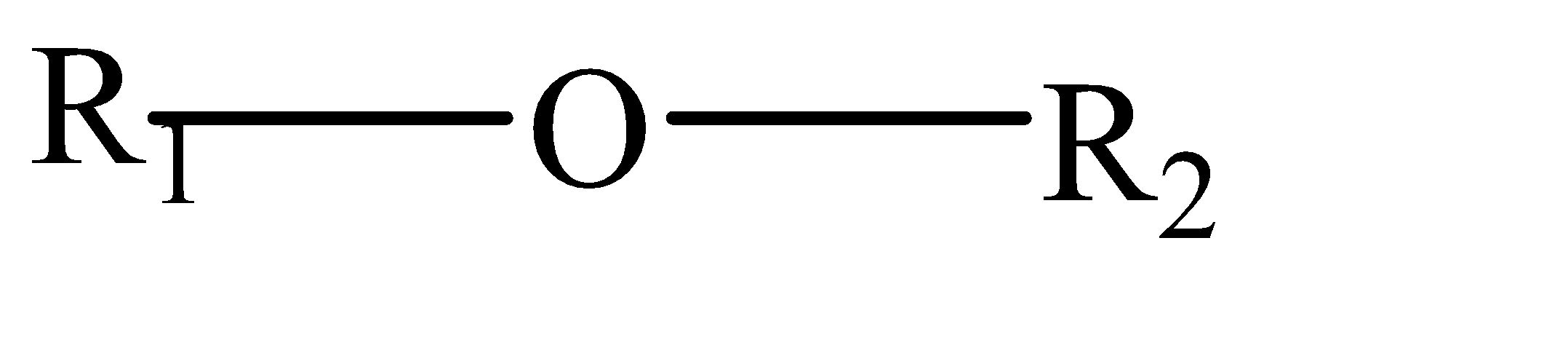
Where R1 and R2 can be aryl as well as alkyl group (same or different).
1-propoxypentane:-
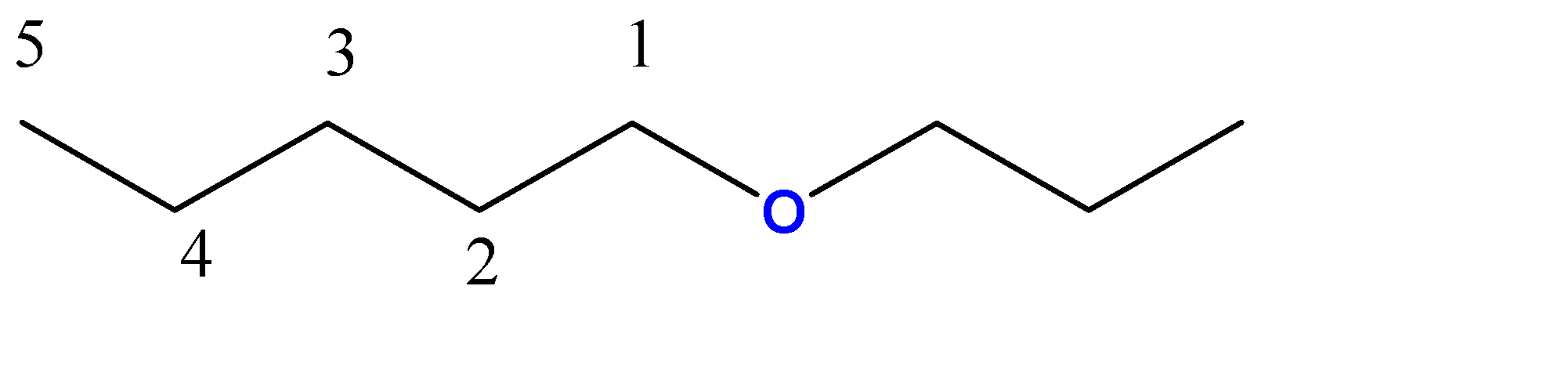
5 carbon atoms chain = pentane. Here, the propoxy group is a 3-carbon chain with an O atom at C1 position.
2-ethoxybutane:-
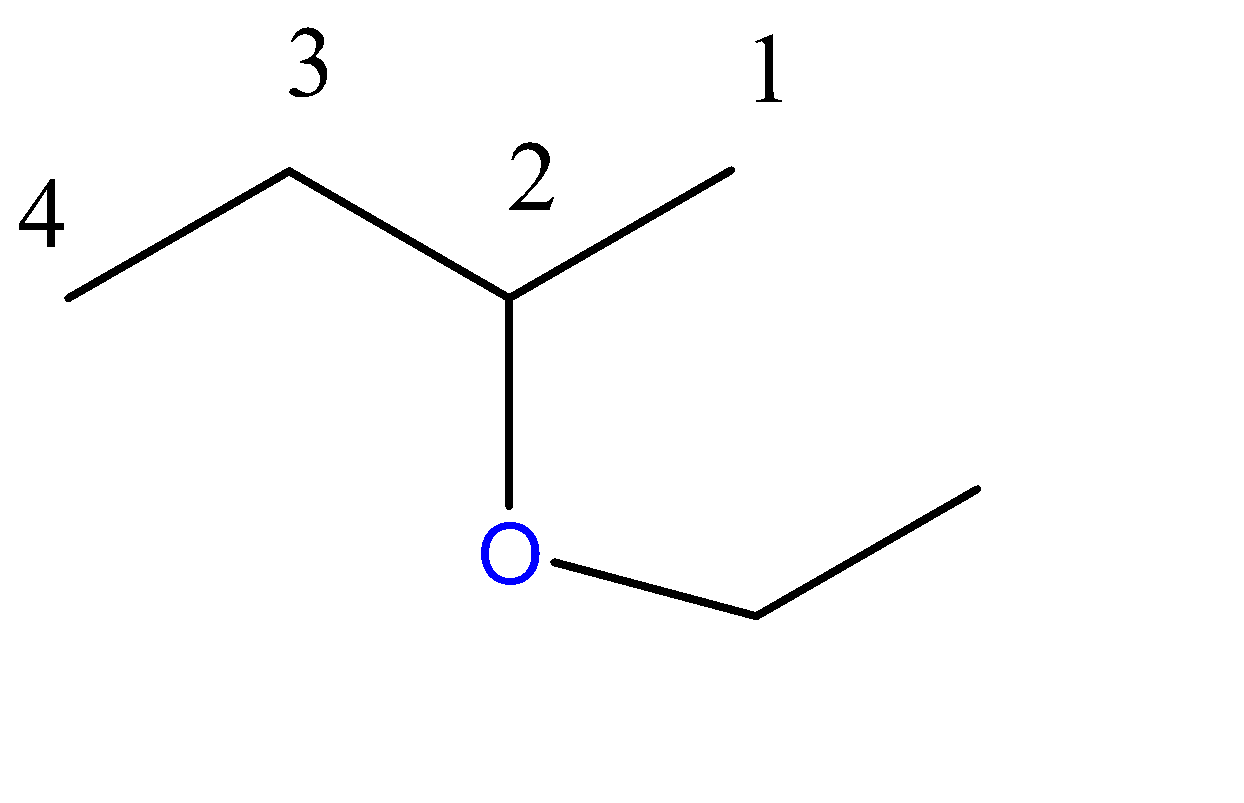
4 carbon atoms chain = butane. Here, the propoxy group is a 3-carbon chain with an O atom at C2 position.
1-methoxy-4-chlorohexane:-
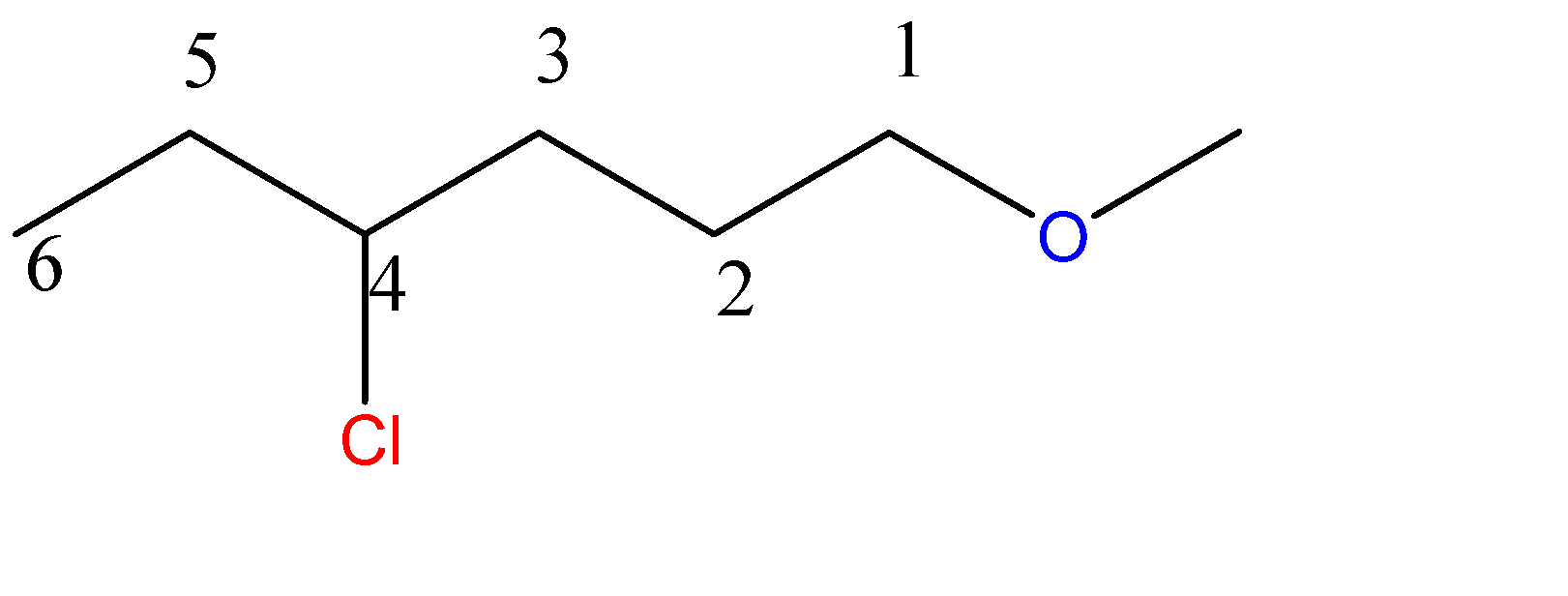
6 carbon atoms chain = hexane. Here, the methoxy group is a 1-carbon chain with an O atom at C1 position and chloro group at C4 position.
3-butoxy-2,4-dimethyloctane:-
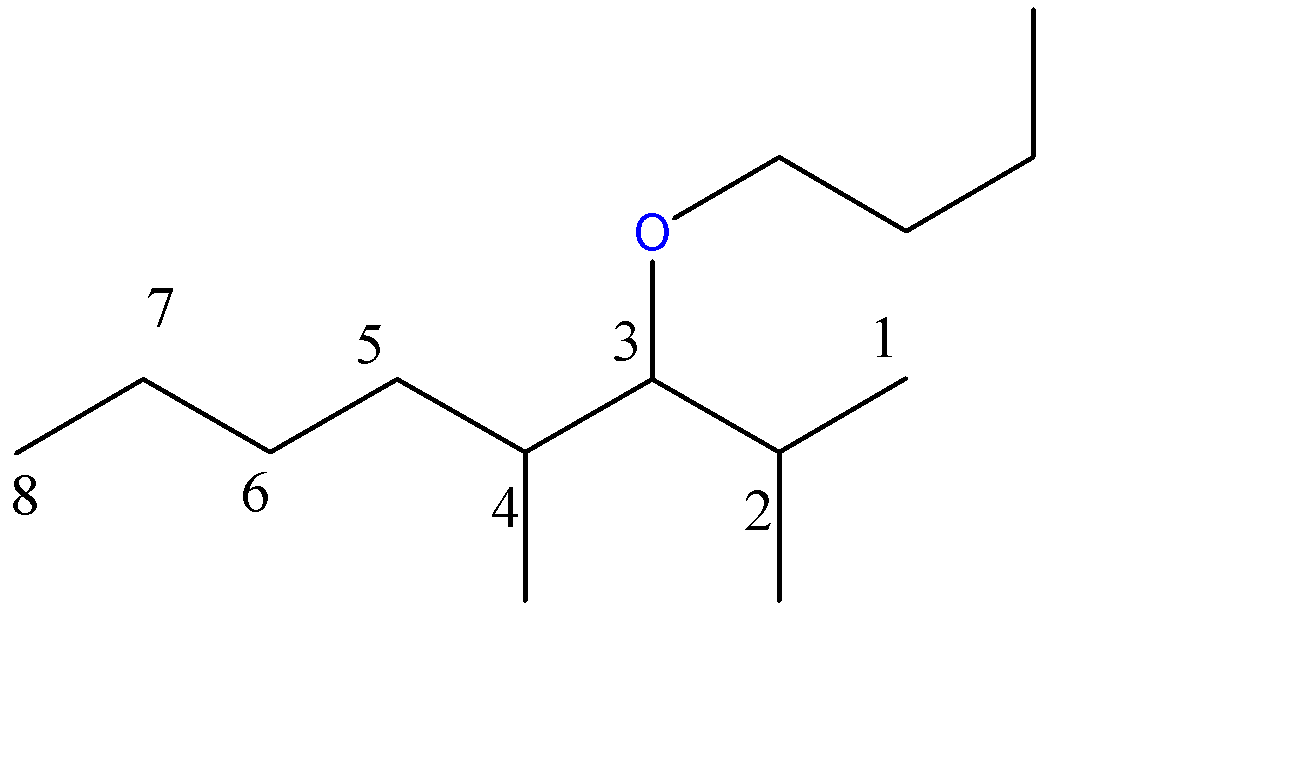
8 carbon atoms chain = octane. Here, the methoxy group is a 1-carbon chain with an O atom at C3 position and 2 methyl groups at C2 and C4 position.
Additional Information:
Ether compounds always contain 1 oxygen atom in it between 2 alkyl/aryl groups. Auto-oxidation of ethers leads to the formation of peroxide compounds. These compounds are used for various purposes especially in fragrant industries. For example: dyes, perfume, scent, fragrant oils and waxes etc.
Note:
Nomenclature of ethers can be done in two ways: a) Common nomenclature prefers to write down the name of all aryl or alkyl groups first(in alphabetical order), followed by the word ‘ether’.
b) According to IUPAC, a substituent with more no. of carbon atoms is chosen as a parent hydrocarbon chain which is used as a suffix whereas the other substituent group attached to O is named as ‘oxy’ which is used as a prefix.
- Always draw the parent hydrocarbon chain at the beginning and give it a number(position) so that later on we can place the right group or substituent on the right carbon position of the parent chain.
Complete step by step solution: Ethers can be categorized as organic compounds where 1 oxygen atom is connected by 2 alkyl or aryl groups. They have the general formula as follows:-
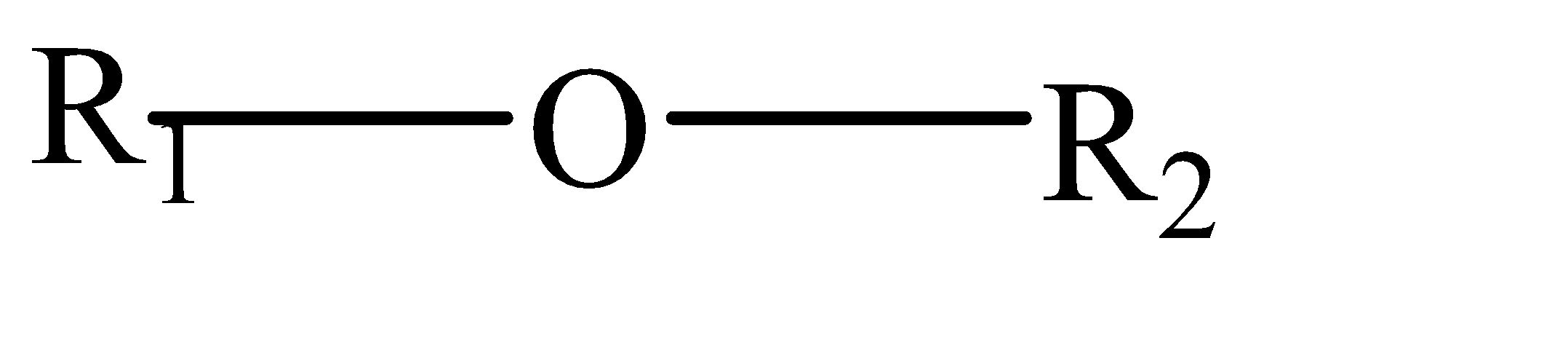
Where R1 and R2 can be aryl as well as alkyl group (same or different).
1-propoxypentane:-

5 carbon atoms chain = pentane. Here, the propoxy group is a 3-carbon chain with an O atom at C1 position.
2-ethoxybutane:-

4 carbon atoms chain = butane. Here, the propoxy group is a 3-carbon chain with an O atom at C2 position.
1-methoxy-4-chlorohexane:-

6 carbon atoms chain = hexane. Here, the methoxy group is a 1-carbon chain with an O atom at C1 position and chloro group at C4 position.
3-butoxy-2,4-dimethyloctane:-

8 carbon atoms chain = octane. Here, the methoxy group is a 1-carbon chain with an O atom at C3 position and 2 methyl groups at C2 and C4 position.
Additional Information:
Ether compounds always contain 1 oxygen atom in it between 2 alkyl/aryl groups. Auto-oxidation of ethers leads to the formation of peroxide compounds. These compounds are used for various purposes especially in fragrant industries. For example: dyes, perfume, scent, fragrant oils and waxes etc.
Note:
Nomenclature of ethers can be done in two ways: a) Common nomenclature prefers to write down the name of all aryl or alkyl groups first(in alphabetical order), followed by the word ‘ether’.
b) According to IUPAC, a substituent with more no. of carbon atoms is chosen as a parent hydrocarbon chain which is used as a suffix whereas the other substituent group attached to O is named as ‘oxy’ which is used as a prefix.
- Always draw the parent hydrocarbon chain at the beginning and give it a number(position) so that later on we can place the right group or substituent on the right carbon position of the parent chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




