
Draw the labelled diagram of Ostwald method for preparation of Nitric Acid and Explain its preparation only by equation?
Answer
504.3k+ views
Hint :We know that nitric acid is a chemical compound made up from the combination of nitrogen, hydrogen and oxygen elements in a specific ratio. It is a highly corrosive mineral acid. Nitric acid is also known as aqua fortis and spirit of niter.
Complete Step By Step Answer:
As we know that the Ostwald’s process is associated with the Haber process. The formation of nitric acid by the catalytic oxidation of ammonia is known as the Ostwald process. In the first step, ammonia is converted into nitrogen oxide. When ammonia reacts with oxygen in the presence of platinum catalysts it forms nitrogen oxide. In the second step, the nitrogen oxide gas converts into nitrogen dioxide, and this nitrogen dioxide gas reacts with water to form nitric acid.
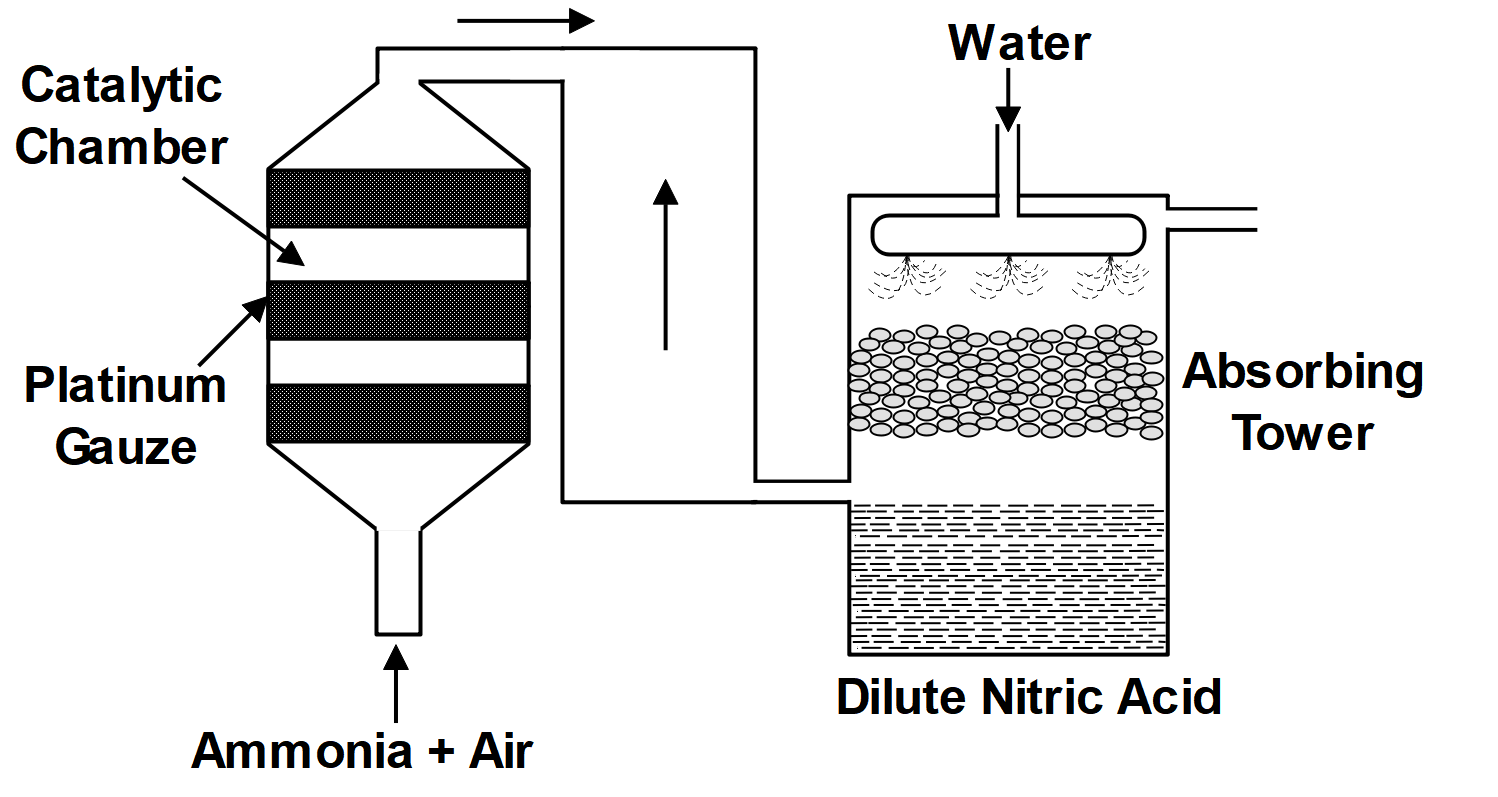
Nitric acid is most commonly manufactured from Ostwald’s process. Ostwald’s process is a chemical process to convert ammonia into nitric acid in two stages. In the beginning ammonia is oxidized to form nitric oxide and nitric dioxide and in the second step the nitrogen dioxide is allowed to dissolve in water and finally nitric acid is formed. Preparation of $ HN{{O}_{3}} $ by the reactions:
$ 4N{{H}_{3}}+5{{O}_{2}}\xrightarrow[750-900]{Pt. guage}4NO+6{{H}_{2}}O $ and further reaction is carried out by \[2NO+{{O}_{2}}\to 2N{{O}_{2}}\xrightarrow{{{H}_{2}}O}2HN{{O}_{3}}+NO.\]
As we can see in the above reactions that first ammonia is converted into nitric oxide and then further with the help of oxygen it is converted into nitric dioxide. The next step involves addition of water in nitric dioxide which finally results in nitric acid. The catalyst used in this reaction is platinum. Overall reaction is exothermic. The Ostwald’s process is considered an important chemical process because it provides a key ingredient for many fertilizers and fertilizer plants.
Note :
Remember that the concentration of nitric acid we use is concentrating on sulphuric acid because sulphuric acid is a dehydrating agent. If we talk about the structure of nitric acid in a gaseous state, nitric acid exists as a planar molecule.
Complete Step By Step Answer:
As we know that the Ostwald’s process is associated with the Haber process. The formation of nitric acid by the catalytic oxidation of ammonia is known as the Ostwald process. In the first step, ammonia is converted into nitrogen oxide. When ammonia reacts with oxygen in the presence of platinum catalysts it forms nitrogen oxide. In the second step, the nitrogen oxide gas converts into nitrogen dioxide, and this nitrogen dioxide gas reacts with water to form nitric acid.

Nitric acid is most commonly manufactured from Ostwald’s process. Ostwald’s process is a chemical process to convert ammonia into nitric acid in two stages. In the beginning ammonia is oxidized to form nitric oxide and nitric dioxide and in the second step the nitrogen dioxide is allowed to dissolve in water and finally nitric acid is formed. Preparation of $ HN{{O}_{3}} $ by the reactions:
$ 4N{{H}_{3}}+5{{O}_{2}}\xrightarrow[750-900]{Pt. guage}4NO+6{{H}_{2}}O $ and further reaction is carried out by \[2NO+{{O}_{2}}\to 2N{{O}_{2}}\xrightarrow{{{H}_{2}}O}2HN{{O}_{3}}+NO.\]
As we can see in the above reactions that first ammonia is converted into nitric oxide and then further with the help of oxygen it is converted into nitric dioxide. The next step involves addition of water in nitric dioxide which finally results in nitric acid. The catalyst used in this reaction is platinum. Overall reaction is exothermic. The Ostwald’s process is considered an important chemical process because it provides a key ingredient for many fertilizers and fertilizer plants.
Note :
Remember that the concentration of nitric acid we use is concentrating on sulphuric acid because sulphuric acid is a dehydrating agent. If we talk about the structure of nitric acid in a gaseous state, nitric acid exists as a planar molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




