
Draw the Lewis structure for carbonate ions?
Answer
538.2k+ views
Hint:Lewis dot structures are also known as electron dot structures. The atomic number of C is 6 and of O is 8.Find the total number of valence electrons in the carbonate ion.
Complete step-by-step answer:So in the question we are asked to draw the Lewis structure of the carbonate ion i.e. $CO_{3}^{2-}$
We know that the Lewis structure is called the Lewis electron dot structure, Lewis dot diagrams, Electron dot formula etc.
In the Lewis dot structure the electrons that contribute to the bonding are drawn as a dot and hence the name for the structure.
There are mainly five steps by which we can draw the complete Lewis structure of any ions, molecule or polyatomic species.
Now let’s briefly discuss those five step and then move on to the solution part of the question:
-So the first step to draw the Lewis structure is to find the total number of valence electrons present in the species that is considered for drawing the Lewis structure.
-Then find the least electronegative atom in the molecule and take them as the central atom.
-Now place the other atoms around the central atom and then put two electrons between two atoms to show the chemical bonding between them.
-After giving two electrons between two atoms, fulfill the octet configuration of the outside atoms i.e. the atoms present around the central atom.
-Finally, check the configuration of the central atom, if the central atom does not possess octet configuration then move a pair of electrons from outside atom and arrange it in between the central atom and the outside atom so that multiple bonds i.e. double or triple bonds are formed.
Now let’s follow the five steps to draw the Lewis structure of carbonate ion.
-In carbonate ion$CO_{3}^{2-}$, there is one C atom and three O atoms along with a charge of -2.Hence the total number of valence electrons present is, $valence\,electrons=4+(3)\times 6+2=24$.Since in C there is 4 valence electrons and in O there is 6.
-In the given compound C is the least electronegative atom and it is taken as the central atom.
Now let’s draw the structure according to the above given steps, fulfill the octet configuration of the outside atoms.
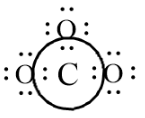
After fulfilling the octet configuration, when we check the configuration of the central atom C there are only 6 valence electrons and hence we move an electron pair from outside atom towards the C atom to form double bond, by which all the atoms will attain the octet configuration.
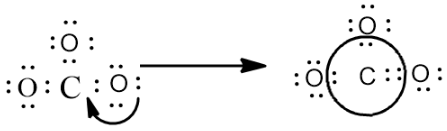
-In this skeleton structure all the atoms have octet configuration.
And the final Lewis structure of $CO_{3}^{2-}$is,
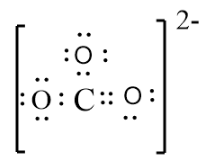
Since there is -2 charge for the carbonate ion it should be represented in the structure.
Note:The -2 charge for the carbonate ion is obtained by the sum of the total formal charge for the structure. The feasible structure possesses low values of formal charges.
The equation for the calculation of formal charge is,
$\text{Formal charge=valence }{{\text{e}}^{\text{-}}}\text{s-non-bonding}\,{{\text{e}}^{\text{-}}}\text{s-}\dfrac{\text{Bonding}\,{{\text{e}}^{\text{-}}}\text{s}}{\text{2}}$
The formal charge calculated for$CO_{3}^{2-}$.
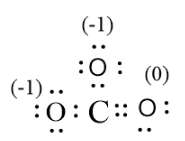
Complete step-by-step answer:So in the question we are asked to draw the Lewis structure of the carbonate ion i.e. $CO_{3}^{2-}$
We know that the Lewis structure is called the Lewis electron dot structure, Lewis dot diagrams, Electron dot formula etc.
In the Lewis dot structure the electrons that contribute to the bonding are drawn as a dot and hence the name for the structure.
There are mainly five steps by which we can draw the complete Lewis structure of any ions, molecule or polyatomic species.
Now let’s briefly discuss those five step and then move on to the solution part of the question:
-So the first step to draw the Lewis structure is to find the total number of valence electrons present in the species that is considered for drawing the Lewis structure.
-Then find the least electronegative atom in the molecule and take them as the central atom.
-Now place the other atoms around the central atom and then put two electrons between two atoms to show the chemical bonding between them.
-After giving two electrons between two atoms, fulfill the octet configuration of the outside atoms i.e. the atoms present around the central atom.
-Finally, check the configuration of the central atom, if the central atom does not possess octet configuration then move a pair of electrons from outside atom and arrange it in between the central atom and the outside atom so that multiple bonds i.e. double or triple bonds are formed.
Now let’s follow the five steps to draw the Lewis structure of carbonate ion.
-In carbonate ion$CO_{3}^{2-}$, there is one C atom and three O atoms along with a charge of -2.Hence the total number of valence electrons present is, $valence\,electrons=4+(3)\times 6+2=24$.Since in C there is 4 valence electrons and in O there is 6.
-In the given compound C is the least electronegative atom and it is taken as the central atom.
Now let’s draw the structure according to the above given steps, fulfill the octet configuration of the outside atoms.

After fulfilling the octet configuration, when we check the configuration of the central atom C there are only 6 valence electrons and hence we move an electron pair from outside atom towards the C atom to form double bond, by which all the atoms will attain the octet configuration.

-In this skeleton structure all the atoms have octet configuration.
And the final Lewis structure of $CO_{3}^{2-}$is,
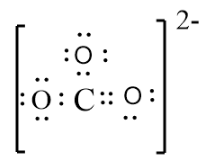
Since there is -2 charge for the carbonate ion it should be represented in the structure.
Note:The -2 charge for the carbonate ion is obtained by the sum of the total formal charge for the structure. The feasible structure possesses low values of formal charges.
The equation for the calculation of formal charge is,
$\text{Formal charge=valence }{{\text{e}}^{\text{-}}}\text{s-non-bonding}\,{{\text{e}}^{\text{-}}}\text{s-}\dfrac{\text{Bonding}\,{{\text{e}}^{\text{-}}}\text{s}}{\text{2}}$
The formal charge calculated for$CO_{3}^{2-}$.
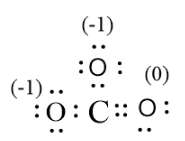
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




