
How do you draw the Lewis structure for the hypobromite ion, $Br{{O}^{-}}$ ?
Answer
558.6k+ views
Hint Lewis dot structures also known as Lewis electron-dot formulas. It uses dots arranged around the chemical symbol for an element to represent the valence electron configuration of the atoms in the element.
Complete step by step solution:
Given in the question:
Hypobromite ion, $Br{{O}^{-}}$
The atomic number of bromine = 35
The number of valence electrons in bromine = 7
The atomic number of oxygen = 8
The number of valence electrons in oxygen = 6
Now there is a negative charge on oxygen which means number of electrons = 7
The Lewis dot structure of hypobromite ion is:
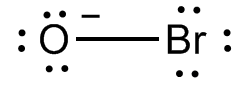
Additional information:
The chemical symbol is used to represent an atom or an element. The chemical symbol of an element usually consists of one or two letters from the Latin alphabet and the first letter is always capital. There are many sources from which the symbols are derived, it includes Greek name of an element or German name etc. For example: the symbol for lead is Pb and it is derived from the Latin name of lead with is plumbum, the symbol for tungsten is W and it is derived from the German name of tungsten which is Wolfram etc.
Note: The possibility to make mistakes is that while drawing the Lewis dot structure of an atom then you may miscount the valence electrons due to which double or triple bonds are drawn incorrectly. Also, don’t forget the lone pairs of an atom.
Complete step by step solution:
Given in the question:
Hypobromite ion, $Br{{O}^{-}}$
The atomic number of bromine = 35
The number of valence electrons in bromine = 7
The atomic number of oxygen = 8
The number of valence electrons in oxygen = 6
Now there is a negative charge on oxygen which means number of electrons = 7
The Lewis dot structure of hypobromite ion is:
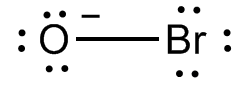
Additional information:
The chemical symbol is used to represent an atom or an element. The chemical symbol of an element usually consists of one or two letters from the Latin alphabet and the first letter is always capital. There are many sources from which the symbols are derived, it includes Greek name of an element or German name etc. For example: the symbol for lead is Pb and it is derived from the Latin name of lead with is plumbum, the symbol for tungsten is W and it is derived from the German name of tungsten which is Wolfram etc.
Note: The possibility to make mistakes is that while drawing the Lewis dot structure of an atom then you may miscount the valence electrons due to which double or triple bonds are drawn incorrectly. Also, don’t forget the lone pairs of an atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




