
Draw the orbital energy diagram for Beryllium.
Answer
493.8k+ views
Hint: Energy of orbitals of hydrogen and hydrogen like atoms which consist of only one electron, depends on the value of principal quantum number only while multi electron atoms depend on both principal quantum number (n) as well as azimuthal quantum number $(l)$.
Complete answer:
Diagrams representing the arrangement of orbitals in increasing order of their energy levels are known as orbital energy diagrams or energy level diagrams. Since beryllium is a multi-electron atom, so some important observations for multi-electron atoms from the energy level diagrams are as follows:
1. The subshell of a particular shell does not have equal energies. For example, 2s and 2p subshells have different energy levels.
2. In a particular shell, a subshell with a lower value of azimuthal quantum number $(l)$ has lower energy i.e., increasing order of energy of subshell can be generalised as $\text{s3. For the same value of n, the energy difference of s and p subshells is relatively small as compared to the energy difference between p and d subshells which is large.
Filling of electrons in orbitals must follow three basic principles which are as follows:
Aufbau principle: It states that electrons are filled in orbitals in the increasing order of the energy associated with it.
Pauli-exclusion principle: It states that no two orbitals can have the same set of quantum numbers and an orbital can have a maximum of 2 electrons and these electrons must be of opposite spin.
Hund’s rule of maximum multiplicity: It deals with the filling of electrons into degenerate orbitals within the same subshell. It states that each orbital must be singly occupied before the filling of electrons takes place.
Now, in the case of Beryllium, to sketch its orbital energy diagram we first need to write its electronic configuration.
Atomic number of Beryllium $=4$
Electronic configuration of Beryllium $=1{{s}^{2}}2{{s}^{2}}$
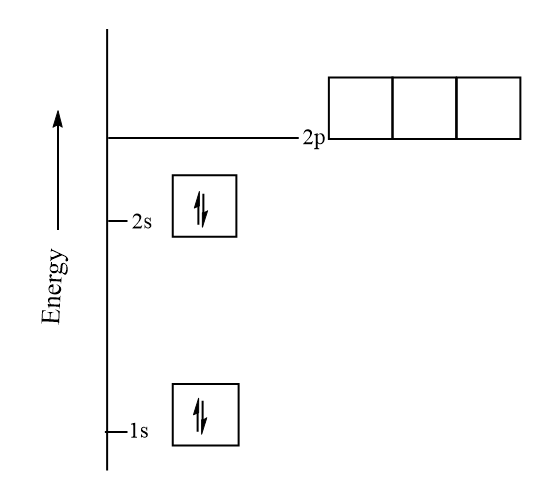
Hence, the orbital energy diagram for Beryllium can be represented as follows:
Note:
It is important to note that in hydrogen atoms, the only force of interaction acting is the coulombic force i.e., force of attraction between the negatively charged electrons and positively charged nucleus. But in case of a multi-electron atom, forces of repulsion among electrons are also present within an electron which leads to large difference in energy levels of subshells.
Complete answer:
Diagrams representing the arrangement of orbitals in increasing order of their energy levels are known as orbital energy diagrams or energy level diagrams. Since beryllium is a multi-electron atom, so some important observations for multi-electron atoms from the energy level diagrams are as follows:
1. The subshell of a particular shell does not have equal energies. For example, 2s and 2p subshells have different energy levels.
2. In a particular shell, a subshell with a lower value of azimuthal quantum number $(l)$ has lower energy i.e., increasing order of energy of subshell can be generalised as $\text{s
Filling of electrons in orbitals must follow three basic principles which are as follows:
Aufbau principle: It states that electrons are filled in orbitals in the increasing order of the energy associated with it.
Pauli-exclusion principle: It states that no two orbitals can have the same set of quantum numbers and an orbital can have a maximum of 2 electrons and these electrons must be of opposite spin.
Hund’s rule of maximum multiplicity: It deals with the filling of electrons into degenerate orbitals within the same subshell. It states that each orbital must be singly occupied before the filling of electrons takes place.
Now, in the case of Beryllium, to sketch its orbital energy diagram we first need to write its electronic configuration.
Atomic number of Beryllium $=4$
Electronic configuration of Beryllium $=1{{s}^{2}}2{{s}^{2}}$
Hence, the orbital energy diagram for Beryllium can be represented as follows:

Note:
It is important to note that in hydrogen atoms, the only force of interaction acting is the coulombic force i.e., force of attraction between the negatively charged electrons and positively charged nucleus. But in case of a multi-electron atom, forces of repulsion among electrons are also present within an electron which leads to large difference in energy levels of subshells.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




