
Draw the $P-V$ diagram for the following cyclic processes:
1. Isothermal expansion from state A to B, isochoric pressure increment from B to C, isothermal contraction from C to D, isobaric contraction from B to A.
2. Isobaric expansion from A to B, isochoric pressure increase from B to C, isobaric compression from C to D, isochoric pressure drop from D to A.
3. Isobaric expansion from A to B, isochoric pressure drop from B to C, isothermal compression form C to A.
Answer
573.9k+ views
Hint: Isothermal process is a process where temperature is constant. Isochoric process is a process where volume is constant. Isobaric process is a process where pressure is constant. You should also have an idea about expansion and contraction of a gas.
Complete step by step answer:
1.
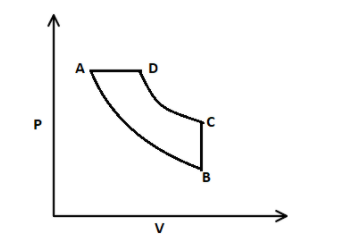
In this process, isothermal expansion takes place from A to B, that means temperature is constant, here expansion means volume is increased and therefore, a curve is formed from A to B. After that, isochoric process takes place, where volume is constant from B to C. Then, isothermal contraction takes place from C to D, where volume is decreased. And then, the isobaric process takes place from D to A, that means constant pressure.
2.
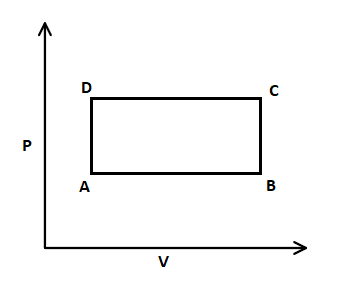
In this $P-V$ graph, firstly isobaric expansion takes place from A to B, where pressure is constant. After that, the isochoric process increases from B to C, where volume is constant, and pressure is increased. Then, isobaric compression takes place from C to D, where pressure is constant, and volume is decreased. Afterwards, isochoric pressure drops from D to A, that means volume is constant and pressure is decreased.
3.
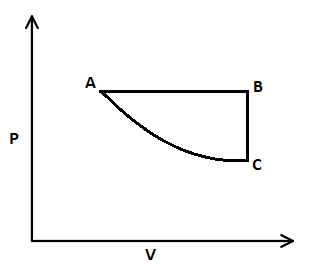
In this $P-V$ graph, firstly isobaric expansion takes place where pressure is constant, and volume is increased. Then, isochoric pressure drops from B to C, that means volume is constant and pressure is decreased. And at last, isothermal compression from C to A, where temperature is constant, and volume is decreased.
Note: cyclic process is defined as the process that starts and returns to the same thermodynamic state.if the cyclic process is clockwise, then work is done on the system ,if the cyclic process is anticlockwise, then work is done by the system.
isothermal process is defined as a thermodynamic process where temperature of the system remains constant. The thermal equilibrium is maintained because transfer of heat occurs very slowly. Some examples of isothermal processes are refrigerator, heat pumps.
Isochoric process is defined as a thermodynamic process where the volume of the system remains constant and it is exemplified by heating and cooling of the contents of a sealed container. Example of an isochoric process is the ideal Otto cycle.
Isobaric process is defined as a thermodynamic process where the pressure on the system remains constant. The constant pressure is obtained, and volume is expanded or contracted.
Complete step by step answer:
1.

In this process, isothermal expansion takes place from A to B, that means temperature is constant, here expansion means volume is increased and therefore, a curve is formed from A to B. After that, isochoric process takes place, where volume is constant from B to C. Then, isothermal contraction takes place from C to D, where volume is decreased. And then, the isobaric process takes place from D to A, that means constant pressure.
2.

In this $P-V$ graph, firstly isobaric expansion takes place from A to B, where pressure is constant. After that, the isochoric process increases from B to C, where volume is constant, and pressure is increased. Then, isobaric compression takes place from C to D, where pressure is constant, and volume is decreased. Afterwards, isochoric pressure drops from D to A, that means volume is constant and pressure is decreased.
3.

In this $P-V$ graph, firstly isobaric expansion takes place where pressure is constant, and volume is increased. Then, isochoric pressure drops from B to C, that means volume is constant and pressure is decreased. And at last, isothermal compression from C to A, where temperature is constant, and volume is decreased.
Note: cyclic process is defined as the process that starts and returns to the same thermodynamic state.if the cyclic process is clockwise, then work is done on the system ,if the cyclic process is anticlockwise, then work is done by the system.
isothermal process is defined as a thermodynamic process where temperature of the system remains constant. The thermal equilibrium is maintained because transfer of heat occurs very slowly. Some examples of isothermal processes are refrigerator, heat pumps.
Isochoric process is defined as a thermodynamic process where the volume of the system remains constant and it is exemplified by heating and cooling of the contents of a sealed container. Example of an isochoric process is the ideal Otto cycle.
Isobaric process is defined as a thermodynamic process where the pressure on the system remains constant. The constant pressure is obtained, and volume is expanded or contracted.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




