
Draw the structure and write the formula of methane.
Answer
528k+ views
Hint: The formula of any organic compound tells us the elements or atoms present in that compound. The formula leads to the structure of organic molecules. Methane is the simplest alkane having one carbon atom, attached with four hydrogen atoms.
Complete answer:
The structure and formula of any organic compound is based on the composition of elements in that compound. The structure can have various types of geometry depending upon the number of covalent bonds in that compound.
Methane is the simplest alkane. It consists of 4 hydrogens and 1 carbon atom. The methane molecule has a carbon in the center. Due to the carbon having only 4 electrons in the outer shell, it can form 4 covalent bonds with hydrogen, due to the presence of only one electron in the valence shell of hydrogen. Therefore, we can write the formula of methane as $C{{H}_{4}}$. This can be made into the structural formula as:
$-\underset{\underset{{}}{\mathop{|}}\,}{\overset{\overset{{}}{\mathop{|}}\,}{\mathop{C}}}\,-$ the 4 single bonds denote the 4 bonded hydrogen atoms.
The structure of methane has a tetrahedral geometry and can be drawn as:
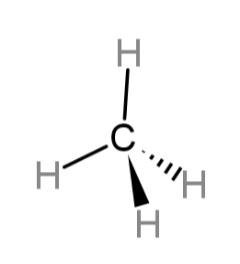
Hence, the structure of methane is tetrahedral and the formula is $C{{H}_{4}}$.
Note:
Covalent bonds are formed when the electrons are shared between atoms, so that the atoms complete their octet. The tendency of carbon to form covalent bonds is due its properties of catenation, which can form long chain structure of organic molecules, as well as tetravalency, due to the absence of 4 electrons to complete the fully filled configuration.
Complete answer:
The structure and formula of any organic compound is based on the composition of elements in that compound. The structure can have various types of geometry depending upon the number of covalent bonds in that compound.
Methane is the simplest alkane. It consists of 4 hydrogens and 1 carbon atom. The methane molecule has a carbon in the center. Due to the carbon having only 4 electrons in the outer shell, it can form 4 covalent bonds with hydrogen, due to the presence of only one electron in the valence shell of hydrogen. Therefore, we can write the formula of methane as $C{{H}_{4}}$. This can be made into the structural formula as:
$-\underset{\underset{{}}{\mathop{|}}\,}{\overset{\overset{{}}{\mathop{|}}\,}{\mathop{C}}}\,-$ the 4 single bonds denote the 4 bonded hydrogen atoms.
The structure of methane has a tetrahedral geometry and can be drawn as:

Hence, the structure of methane is tetrahedral and the formula is $C{{H}_{4}}$.
Note:
Covalent bonds are formed when the electrons are shared between atoms, so that the atoms complete their octet. The tendency of carbon to form covalent bonds is due its properties of catenation, which can form long chain structure of organic molecules, as well as tetravalency, due to the absence of 4 electrons to complete the fully filled configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




