
Draw the structure of Cyclopropanone oxime.
Answer
587.7k+ views
Hint: In the structure of the Cyclopropanone oxime there is a presence of a cyclopropane ring and an oxime functional group.
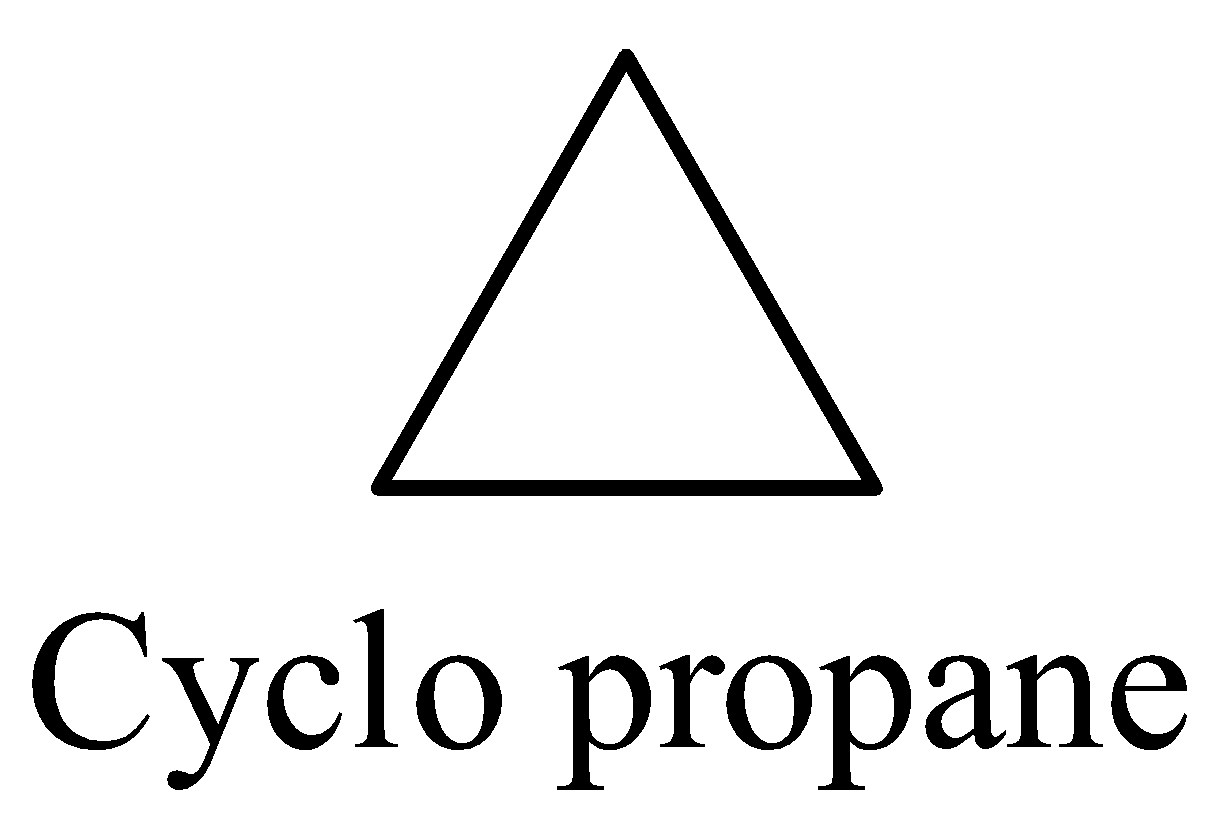
Structure of the cyclopropane ring is as follows.
Representation of an oxime functional group is as follows.
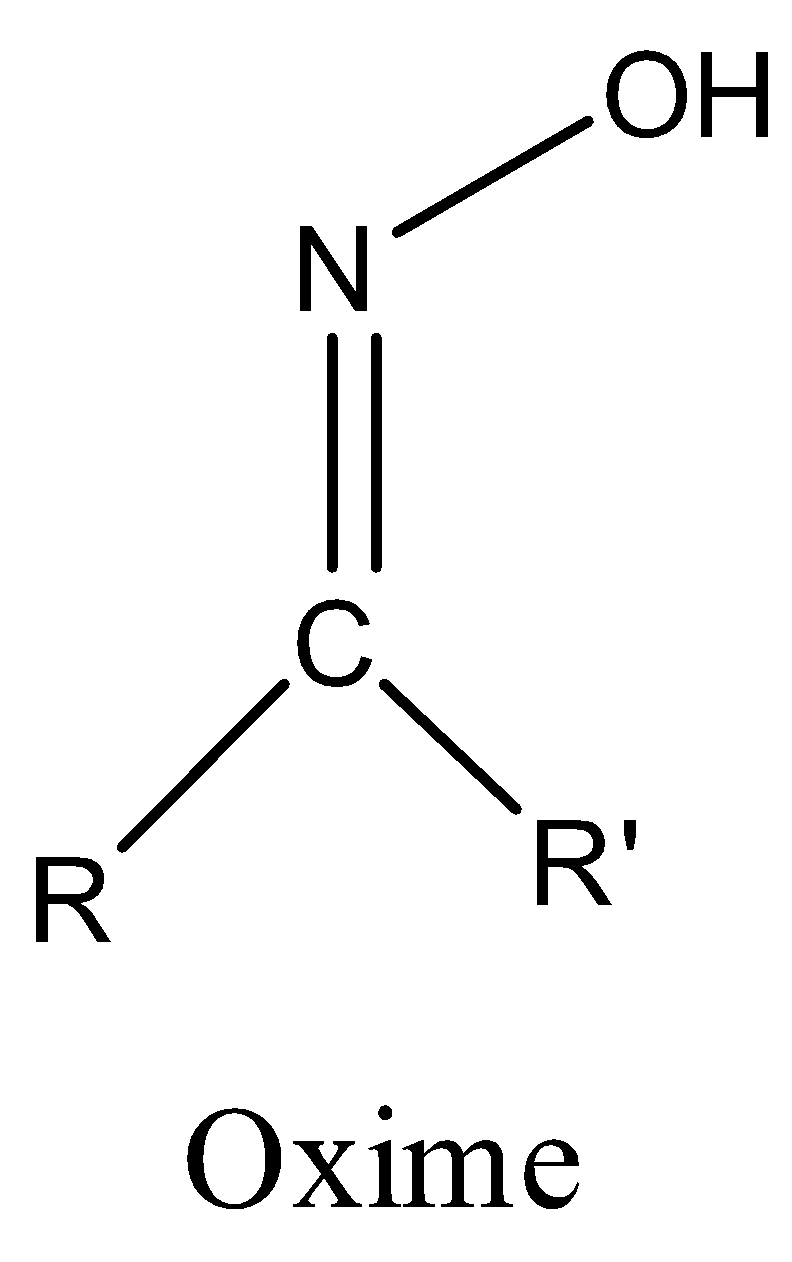
If R = alkyl and R’ = H in the above structure then it is called aldoxime and
R = alkyl and R’ = alkyl in the above structure then it is called ketoxime.
Complete step by step answer:
- In the question it is asked to draw the structure of the cyclopropanone oxime.
- The reaction of cylcopropanone with hydroxylamine forms cyclopropanone oxime.
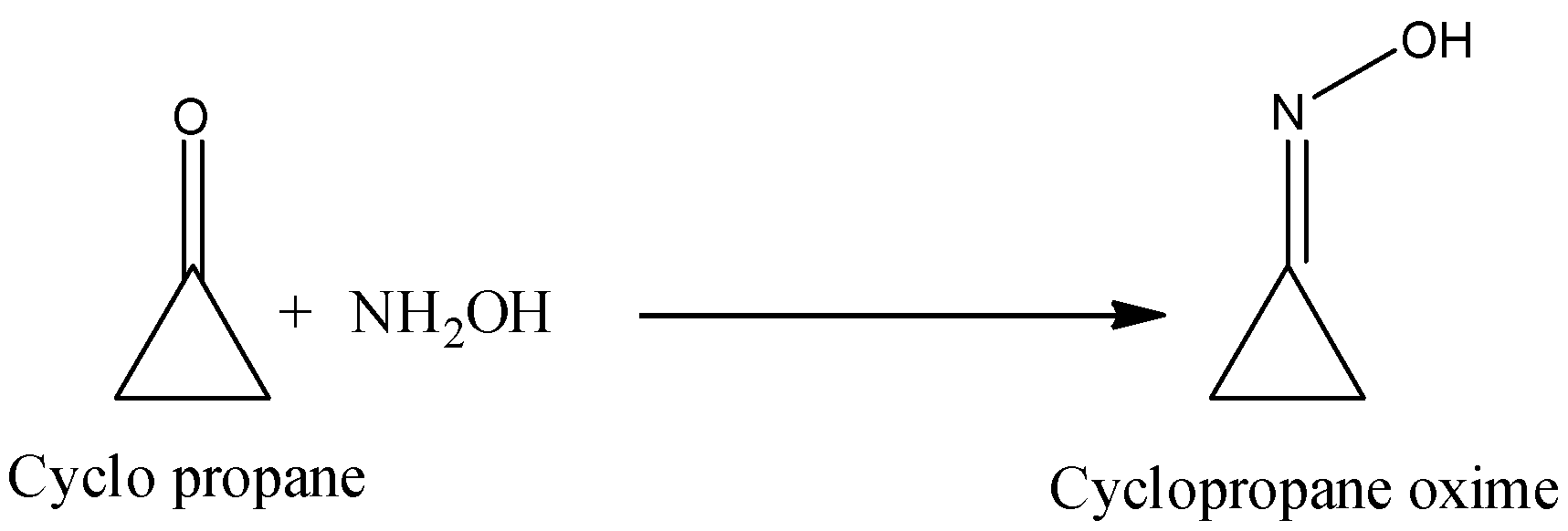
-One of the carbon atoms in a cyclopropane ring attached with N with the help of a double bond in the structure of the cyclopropanone oxime and there is a presence of one –OH group.
Additional information:
- The molecular formula of cyclopropanone oxime is \[{{C}_{3}}{{H}_{3}}NO\].
- Aldehydes react with hydroxylamine and forms aldoximes and ketones react with hydroxyl amine and forms ketoximes as the products.
-The reaction mechanism of aldehydes or ketones with hydroxylamine is as follows.
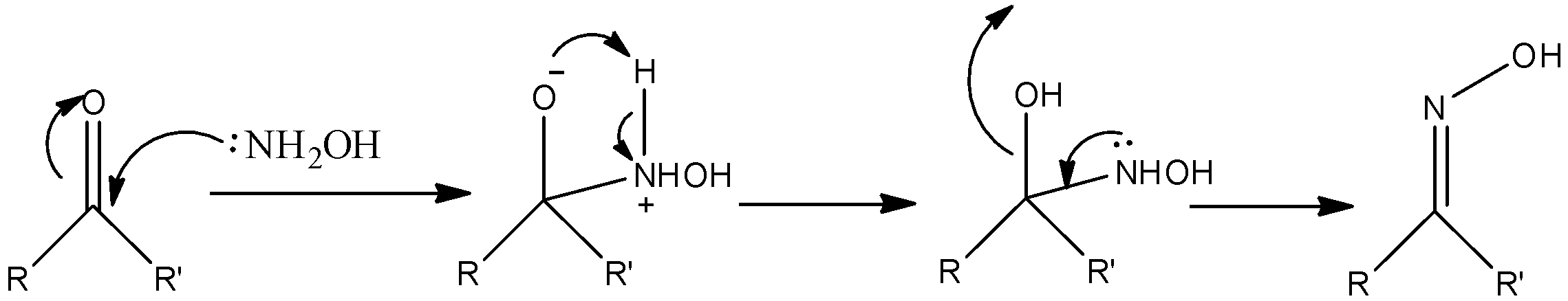
- Where R = alkyl and R’ = alkyl for ketone.
- where R =alkyl and R’ = hydrogen for aldehyde.
Note: The nucleophilic character of the nitrogen in the hydroxylamine is improved by the presence of the oxygen in ketone or aldehyde. Consecutive transfer of protons allows the elimination of water and forms oxime as the product. Oximes usually form a mixture of syn or anti geometric isomers.
Structure of the cyclopropane ring is as follows.

Representation of an oxime functional group is as follows.

If R = alkyl and R’ = H in the above structure then it is called aldoxime and
R = alkyl and R’ = alkyl in the above structure then it is called ketoxime.
Complete step by step answer:
- In the question it is asked to draw the structure of the cyclopropanone oxime.
- The reaction of cylcopropanone with hydroxylamine forms cyclopropanone oxime.
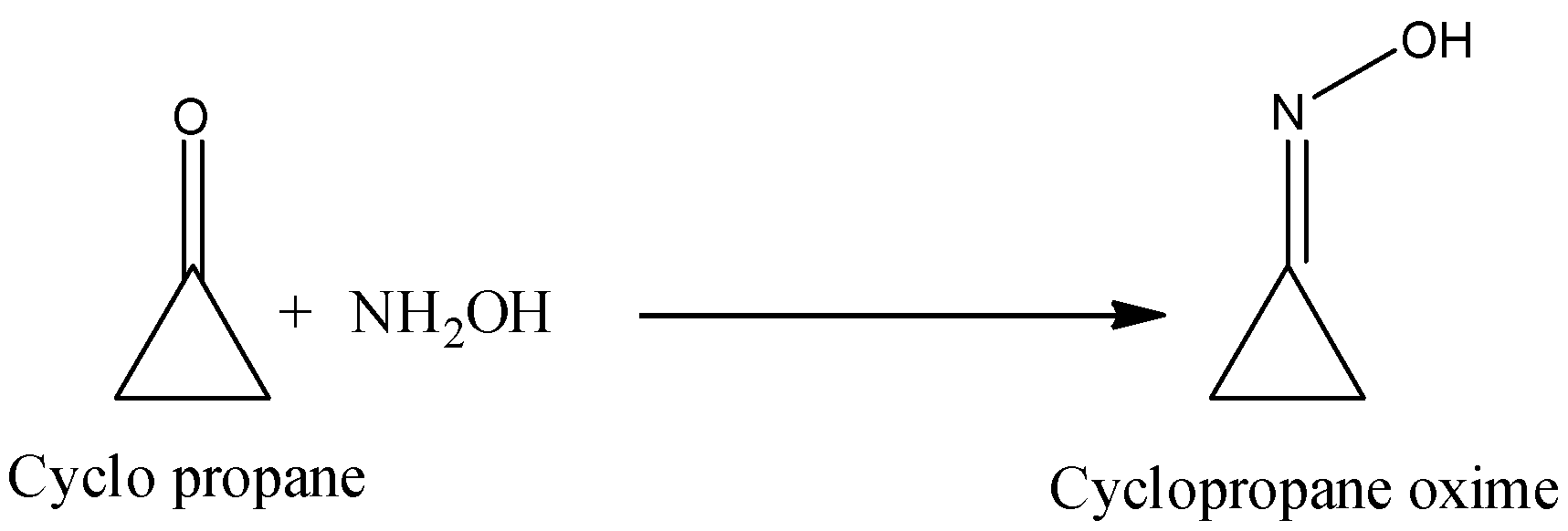
-One of the carbon atoms in a cyclopropane ring attached with N with the help of a double bond in the structure of the cyclopropanone oxime and there is a presence of one –OH group.
Additional information:
- The molecular formula of cyclopropanone oxime is \[{{C}_{3}}{{H}_{3}}NO\].
- Aldehydes react with hydroxylamine and forms aldoximes and ketones react with hydroxyl amine and forms ketoximes as the products.
-The reaction mechanism of aldehydes or ketones with hydroxylamine is as follows.

- Where R = alkyl and R’ = alkyl for ketone.
- where R =alkyl and R’ = hydrogen for aldehyde.
Note: The nucleophilic character of the nitrogen in the hydroxylamine is improved by the presence of the oxygen in ketone or aldehyde. Consecutive transfer of protons allows the elimination of water and forms oxime as the product. Oximes usually form a mixture of syn or anti geometric isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




