
Draw the structure of $ {{H}_{2}}S{{O}_{5}},\text{ }Cr{{O}_{5}} $ and $ NO $ also mention the oxidation state of each atom.
Answer
528.3k+ views
Hint: We know that the caro's acid is also called Peroxymonosulfuric acid. At time of calculating the oxidation state we have to check whether the molecule contains a peroxide bond or not. If the molecule contains a peroxide bond then the oxidation state of the central atom is going to change.
Complete step by step solution:
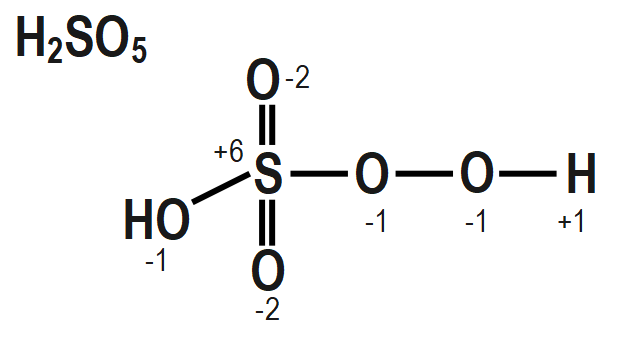
In the question it is given that to calculate the oxidation number of sulphur in Caro's acid. To calculate the oxidation number of sulphur in Caro's acid we have to draw the structure of it and it is as follows
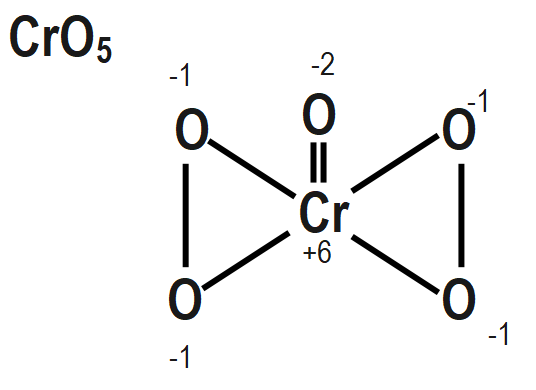
To find out the peroxide bonds in a compound we can consider the oxidation number of a central metal in a compound and if the oxidation number of a central metal in a compound that we are calculating by simple method is coming out to be greater than the maximum possible oxidation state of that central metal then that compound must contain peroxide bonds. These peroxide bonds can compensate for the oxidation number of that central metal to a lower value that may be equal to the maximum possible oxidation state of that metal.
The structure of $ {{H}_{2}}S{{O}_{5}},\text{ }Cr{{O}_{5}} $ and $ NO $ also mention the oxidation state of each atom is given as follows as:
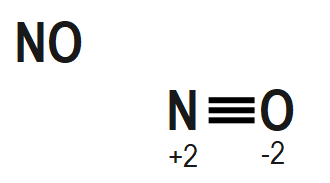
Note:
Remember that When we are going to calculate the oxidation number of an element in a peroxide molecule we are supposed to consider the oxidation number of oxygen in the peroxide bond is $ -1. $ If we consider the oxidation number of an oxygen in the peroxide bond as $ -2 $ then it will be wrong.
Complete step by step solution:
In the question it is given that to calculate the oxidation number of sulphur in Caro's acid. To calculate the oxidation number of sulphur in Caro's acid we have to draw the structure of it and it is as follows
To find out the peroxide bonds in a compound we can consider the oxidation number of a central metal in a compound and if the oxidation number of a central metal in a compound that we are calculating by simple method is coming out to be greater than the maximum possible oxidation state of that central metal then that compound must contain peroxide bonds. These peroxide bonds can compensate for the oxidation number of that central metal to a lower value that may be equal to the maximum possible oxidation state of that metal.
The structure of $ {{H}_{2}}S{{O}_{5}},\text{ }Cr{{O}_{5}} $ and $ NO $ also mention the oxidation state of each atom is given as follows as:


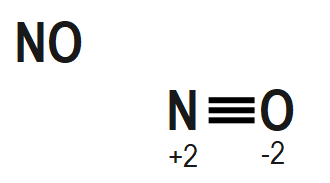
Note:
Remember that When we are going to calculate the oxidation number of an element in a peroxide molecule we are supposed to consider the oxidation number of oxygen in the peroxide bond is $ -1. $ If we consider the oxidation number of an oxygen in the peroxide bond as $ -2 $ then it will be wrong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




