
Draw the structure of Methyl hemiacetal of formaldehyde.
Answer
518.7k+ views
Hint: A hemiacetal is a compound that contains both alcohol and ether and is attached to the same carbon. Hydrogen occupies the fourth bonding position. An aldehyde is used to make a hemiacetal. A hemiketal is a compound that contains an alcohol and an ether, as well as two other carbons.
Complete answer:
A hemiacetal is a compound that contains both alcohol and ether and is attached to the same carbon. Hydrogen occupies the fourth bonding position. An aldehyde is used to make a hemiacetal. A hemiketal is a compound that contains an alcohol and an ether, as well as two other carbons.
HCHO is the formula for formaldehyde, a naturally occurring organic compound. The pure compound is a pungent-smelling colourless gas that naturally polymerizes into paraformaldehyde, so it's held in an aqueous solution. It is the most simple of aldehydes.
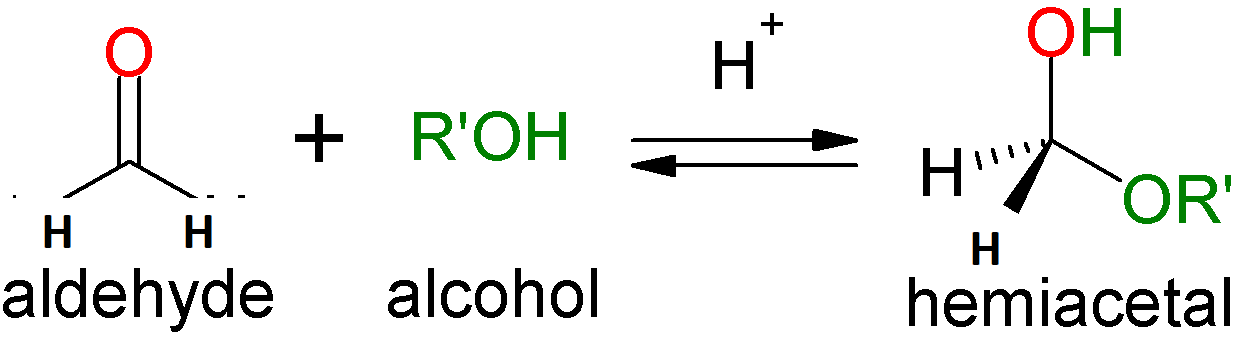
The methyl hemiacetal of formaldehyde would have a single C-atom in the centre of its structure.
Place four bonds around the C-atom after that.
Then add an H-H atom to the two bonds.
So, on the row third, add an OH atom.
Then $OC{H_3}$ will be attached on the last one.
Note:
A hemiacetal is a compound that contains both alcohol and ether and is attached to the same carbon. Hydrogen occupies the fourth bonding position. An aldehyde is used to make a hemiacetal. A hemiketal is a compound that contains an alcohol and an ether, as well as two other carbons.
Complete answer:
A hemiacetal is a compound that contains both alcohol and ether and is attached to the same carbon. Hydrogen occupies the fourth bonding position. An aldehyde is used to make a hemiacetal. A hemiketal is a compound that contains an alcohol and an ether, as well as two other carbons.
HCHO is the formula for formaldehyde, a naturally occurring organic compound. The pure compound is a pungent-smelling colourless gas that naturally polymerizes into paraformaldehyde, so it's held in an aqueous solution. It is the most simple of aldehydes.

The methyl hemiacetal of formaldehyde would have a single C-atom in the centre of its structure.
Place four bonds around the C-atom after that.
Then add an H-H atom to the two bonds.
So, on the row third, add an OH atom.
Then $OC{H_3}$ will be attached on the last one.
Note:
A hemiacetal is a compound that contains both alcohol and ether and is attached to the same carbon. Hydrogen occupies the fourth bonding position. An aldehyde is used to make a hemiacetal. A hemiketal is a compound that contains an alcohol and an ether, as well as two other carbons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




